Inhibitory Activity of Plant Essential Oils against E. coli 1-Deoxy-d-xylulose-5-phosphate reductoisomerase
Abstract
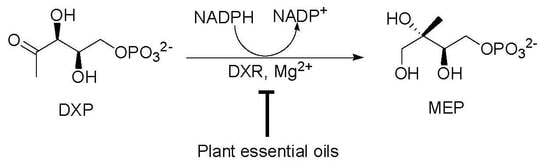
:1. Introduction
2. Results
2.1. Isolation of Plant EOs
2.2. Photometric Assay of the DXR Inhibitory Activity of Plant EOs
2.3. GC and GC-MS Analyses of the Active EOs and DXR Inhibition of the Major Components
3. Material and Methods
3.1. Materials
3.2. Isolation of Plant EOs
3.3. Preparation of Recombinant E. coli DXR
3.4. DXR Inhibitory Activity Determination Using Photometric Assay
3.5. GC and GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burt, S. EOs: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F. Antibacterial and antifungal activities of EOs. In Lipids and EOs as Antimicrobial Agents; Thormar, H., Ed.; Wiley: Huboken, NJ, USA, 2011; pp. 255–306. [Google Scholar]
- Al-Sayed, E. Unearthing the chemical composition of Taxodium distichum (L.) Rich. leaf essential oil and its antimicrobial activity. Ind. Crop. Prod. 2018, 126, 76–82. [Google Scholar] [CrossRef]
- Knezevic, P.; Aleksic, V.; Simin, N.; Svircev, E.; Petrovic, A.; Mimica-Dukic, N. Antimicrobialactivity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016, 178, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of the Cuminum cyminum L. and Carum carvi L. EOs. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Shanmugaiah, V.; Iacobellis, N.S. Antibacterial activity of essential oil components and their potential use in seed disinfection. J. Agric. Food Chem. 2009, 57, 9454–9461. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Antimicrobial activities of gaseous essential oils against Listeria monocytogenes on a laboratory medium and radish sprouts. Int. J. Food Microbiol. 2018, 265, 49–54. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, I.C.; Santos Frazao, G.G.; Blank, A.F.; De Aquino Santana, L.C. Myrcia ovata Cambessedes essential oils: A proposal for a novel natural antimicrobial against foodborne bacteria. Microb. Pathog. 2016, 99, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 EOs of different origin as functional antioxidants, antiradicals and antimicrobials in food. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Jiang, N.; Qin, L.Y.; Chen, Q.P.; Liu, W. Antimicrobial activity of 10 edible spice EOs against phytopathogenic fungi. Plant Protect. Sci. 2012, 38, 104–107. (In Chinese) [Google Scholar]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Sakthivel, R.; Nisha, S.A.; Suganthy, N.; Pandian, S.K. Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis. Arch. Pharm. Res. 2013, 36, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. Methylerythritol Phosphate Pathway. In Comprehensive Natural Products II, Chemistry and Biology; Mander, L., Liu, H.W., Eds.; Elsevier: New York, NY, USA, 2010; Volume 1, pp. 517–555. [Google Scholar]
- Munos, J.W.; Pu, X.; Mansoorabadi, S.O.; Kim, H.J.; Liu, H.W. A secondary kinetic isotope effect study of the 1-deoxy-d-xylulose-5-phosphate reductoisomerase catalyzed reaction: Evidence for a retroaldol-aldol rearrangement. J. Am. Chem. Soc. 2009, 131, 2048–2049. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.A.; Sathyamoorthy, B.; Eletsky, A.; Szyperski, T.; Murkin, A.S. Highly precise measurement of kinetic isotope effects using 1H-detected 2D [13C,1H]-HSQC NMR spectroscopy. J. Am. Chem. Soc. 2012, 134, 20589–20592. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Sun, W.; Qin, W.; Gao, W.Y. Mechanistic insights into 1-deoxy-d-xylulose 5-phosphate reductoisomerase, a key enzyme of the MEP terpenoid biosynthetic pathway. FEBS J. 2013, 280, 5896–5905. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, H.; Wiesner, J.; Sanderbrand, S.; Altincicek, B.; Weidemeyer, C.; Hintz, M.; Türbachova, I.; Eberl, M.; Zeidler, J.; Lichtenthaler, H.K.; et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 1999, 285, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Missinou, M.A.; Borrmann, S.; Schindler, A.; Issifou, S.; Adegnika, A.A.; Matsiegui, P.B. Fosmidomycin for malaria. Lancet 2002, 360, 1941–1942. [Google Scholar] [CrossRef]
- Jackson, E.R.; Dowd, C.S. Inhibition of 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR): A review of the synthesis and biological evaluation of recent inhibitors. Curr. Topics Med. Chem. 2012, 12, 706–728. [Google Scholar] [CrossRef]
- Deng, L.; Sundriyal, S.; Rubio, V.; Shi, Z.-Z.; Song, Y. Coordination chemistry based approach to lipophilic inhibitors of 1-deoxy-d-xylulose-5-phosphate reductoisomerase. J. Med. Chem. 2009, 52, 6539–6542. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Yassin, M.; Prakash, S.; Safi, N.; Agamid, M.; Lauw, S.; Ostrozhenkova, E.; Bacher, A.; Rohdich, F.; Eisenreich, W.; et al. Anti-malarial drug targets: Screening for inhibitors of 2C-methyl-d-erythritol 4-phosphate synthase (IspC protein) in Mediterranean plants. Phytomedicine 2007, 14, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Yue, Q.; Zhang, D.D.; Li, H.; Yang, S.Q.; Gao, W.Y. Antimicrobial mechanism of theaflavins: They target 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Sci. Rep. 2016, 6, 38945. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Liu, H.; Tian, F.L.; Li, F.F.; Li, H.; Gao, W.Y. Inhibition of green tea and the catechins against 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Fitoterapia 2016, 113, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Hui, X.; Hua, S.H.; Wu, Q.Q.; Li, H.; Gao, W.Y. Antimicrobial mechanism of epigallocatechin gallate and gallocatechin gallate: They target 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Arch. Biochem. Biophys. 2017, 622, 1–8. [Google Scholar] [CrossRef]
- Hui, X.; Yan, G.; Tian, F.L.; Li, H.; Gao, W.Y. Antimicrobial mechanism of the major active essential oil compounds and their structure–activity relationship. Med. Chem. Res. 2017, 26, 442–449. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Wang, H.; Yang, S.Q.; Gao, W.Y. An improved preparation of D-glyceraldehyde 3-phosphate and its use in the synthesis of 1-deoxy-Dxylulose 5-phosphate. Helv. Chim. Acta 2010, 93, 1745–1750. [Google Scholar] [CrossRef]
- Boyle, W. Spices and essential oils as preservatives. Am. Perfum. Essent. Oil Rev. 1955, 66, 25–28. [Google Scholar]
- Tinivella, F.; Hirata, L.M.; Celan, M.A.; Wright, S.A.I.; Amein, T.; Schmitt, A.; Koch, E.; van der Wolf, J.M.; Groot, S.P.C.; Stephan, D.; et al. Control of seed-borne pathogens on legumes by microbial and other alternative seed treatments. Eur. J. Plant Pathol. 2009, 123, 139–151. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wendakoon, C.N.; Sakaguchi, M. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Condo, C.; Anacarso, I.; Sabia, C.; Iseppi, R.; Anfelli, I.; Forti, L.; De Niederhausern, S.; Bondi, M.; Messi, P. Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 2018, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Tursi, F.; Vitali, C.; Miccolis, V.; Candido, V. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 2000, 7, 239–243. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, I.; Menon, A.N. Comparative chemical composition and antimicrobial activity of fresh & dry ginger oils. Int. J. Cur. Pharm. Res. 2010, 2, 40–43. [Google Scholar]
- Tao, F.Y.; Zhang, X.M.; Yu, J.; Ma, R.Y. Progress on mechanism of antimicrobial activity of tea tree oil. Zhongguo Kangshengsu Zazhi 2006, 31, 261–266. (In Chinese) [Google Scholar]
Sample Availability: Samples of all the EOs and individual EO compounds are available from the authors. |



| EOs | IC50 (μg/mL) |
|---|---|
| Zanbthoxylum bungeanum (ZB) | 78.05 ± 3.37 |
| Schizonepetae tenuifoliae (ST) | 65.32 ± 2.54 |
| Thymus quinquecostatus (TQ) | 59.48 ± 3.11 |
| Origanum vulgare (OV) | 48.37 ± 1.07 |
| Eugenia caryophyllata (EC) | 37.25 ± 2.58 |
| Fosmidomycin * | 0.038 ± 0.014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Zhu, B.-R.; Tian, F.-L.; Hui, X.; Li, H.; Li, Y.-M.; Gao, W.-Y. Inhibitory Activity of Plant Essential Oils against E. coli 1-Deoxy-d-xylulose-5-phosphate reductoisomerase. Molecules 2019, 24, 2518. https://doi.org/10.3390/molecules24142518
Yan G, Zhu B-R, Tian F-L, Hui X, Li H, Li Y-M, Gao W-Y. Inhibitory Activity of Plant Essential Oils against E. coli 1-Deoxy-d-xylulose-5-phosphate reductoisomerase. Molecules. 2019; 24(14):2518. https://doi.org/10.3390/molecules24142518
Chicago/Turabian StyleYan, Ge, Bo-Rong Zhu, Fang-Lin Tian, Xian Hui, Heng Li, Yi-Ming Li, and Wen-Yun Gao. 2019. "Inhibitory Activity of Plant Essential Oils against E. coli 1-Deoxy-d-xylulose-5-phosphate reductoisomerase" Molecules 24, no. 14: 2518. https://doi.org/10.3390/molecules24142518