A Citrus Peel Waste Biorefinery for Ethanol and Methane Production
Abstract
:1. Introduction
2. Results and Discussion
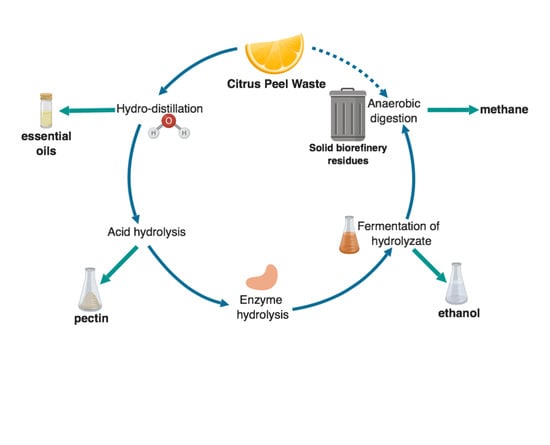
2.1. The CPW Biorefinery Strategy
2.2. Ethanol Production
2.2.1. Ethanol Production Using CPW Hydrolyzates Obtained through Acid Hydrolysis
2.2.2. Optimization of the Fermentation Process
2.3. Anaerobic Digestion of Biorefinery Residues
2.3.1. Methane Production Using BR, CPW, and DCPW
2.3.2. Biomethane Production Using Different Initial Quantities of CPW
3. Materials and Methods
3.1. Citrus Peel Waste
3.2. Pretreatment of Citrus Peel Waste
3.2.1. Extraction of Essential Oils and Pectin
3.2.2. Enzyme Hydrolysis
3.3. Ethanol Fermentations
3.4. Anaerobic Digestion Experiments
3.5. Analyses
3.5.1. Ethanol Concentration
3.5.2. Reducing Sugars
3.5.3. Total and Volatile Solids
3.5.4. Biogas Composition
3.5.5. Volatile Fatty Acids (VFAs)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kosseva, M.R. Sources, characterization, and composition of food industry wastes. In Food Industry Wastes, 1st ed.; Kosseva, M.R., Webb, C., Eds.; Elsevier Inc.: London, UK, 2013; pp. 37–60. [Google Scholar]
- FAO. Citrus Fruit Statistics 2015; FAO: Rome, Italy, 2016; p. 53. [Google Scholar]
- Marin, F.R.; Soler-Rivas, C.; Benavente-Garcia, O.; Castillo, J.; Perez-Alvarez, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Wilkins, M.R. Effect of orange peel oil on ethanol production by Zymomonas mobilis. Biomass Bioenergy 2009, 33, 538–541. [Google Scholar] [CrossRef]
- Negro, V.; Bernardo, R.; Fino, D. Recovery of energy from orange peels through anaerobic digestion and pyrolysis processes after D-limonene extraction. Waste Biomass Valoriz. 2018, 9, 1331–1337. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of citrus by-products into value added products. Waste Biomass Valoriz. 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Citrus essential oils and their influence on the anaerobic digestion process: An overview. Waste Manag. 2014, 34, 2063–2079. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.S.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Siles, J.A.; Thompson, I.P. Succinic acid production from orange peel and wheat straw by batch fermentations of Fibrobacter succinogenes S85. Appl. Microbiol. Biotechnol. 2010, 88, 671–678. [Google Scholar] [CrossRef]
- Martin, M.A.; Siles, J.A.; Chica, A.F.; Martin, A. Biomethanization of orange peel waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Siles, J.A.; Vargas, F.; Gutierrez, M.C.; Chica, A.F.; Martin, M.A. Integral valorisation of waste orange peel using combustion, biomethanisation and co-composting technologies. Bioresour. Technol. 2016, 211, 173–182. [Google Scholar] [CrossRef]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, D.; Zhao, S.; Zhu, H.; Kenttamma, H.I.; Abu-Omar, M.M. Renewable thermoset polymers based on lignin and carbohydrate derived monomers. Green Chem. 2018, 20, 1131–1138. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Swain, M.R. Bioethanol production from corn and wheat: Food, fuel and future. In Bioethanol Production from Food Crops, 1st ed.; Ray, R.C., Ramachandran, C., Eds.; Elsevier Inc.: London, UK, 2019; pp. 45–49. [Google Scholar]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced ethanol production from Kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresour. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem. 2007, 42, 1614–1619. [Google Scholar] [CrossRef]
- Forgacs, G.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J.; Hovath, I.S. Methane production from citrus wastes: Process development and cost estimation. J. Chem. Technol. Biotechnol. 2011, 87, 250–255. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Oberoi, H.S.; Dhaliwal, S.S.; Babbar, N.; Kaur, U.; Nanda, D.; Kumar, D. Ethanol production from Kinnow mandarin (Citrus reticulata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia kudriavzevii strain. Ann. Microbiol. 2012, 62, 655–666. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, J.H.; Wi, S.G.; Kim, K.H.; Bae, H.J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping treatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L.; Saida, L.; Abeykoon, J.P. Ethanol production from orange peels: Two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene production from ethanol: A review and techno-sconomical evaluation. ChemBioEng Rev. 2017, 2, 75–91. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Alisaraei, A.; Hosseini, S.H.; Ghobadian, B.; Motevali, A. Biofuel production from citrus wastes: A feasibility study in Iran. Renew. Sustain. Energy Rev. 2017, 69, 1100–1112. [Google Scholar] [CrossRef]
- Kyriakou, M.; Chatziiona, V.K.; Costa, C.N.; Kallis, M.; Koutsokeras, L.; Constantinides, G.; Koutinas, M. Biowaste-based biochar: A new strategy for fermentative bioethanol overproduction via whole-cell immobilization. Appl. Energy 2019, 242, 480–491. [Google Scholar] [CrossRef]
- Tavares, B.; de Almeida Felipe, M.G.; dos Santos, J.C.; Pereira, F.M.; Gomes, S.D.; Sene, L. An experimental and modeling approach for ethanol production by Kluyveromyces marxianus in stirred tank bioreactor using vacuum extraction as a strategy to overcome product inhibition. Renew. Energy 2019, 131, 261–267. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crepeau, M.J.; Sorensen, S.O.; Ralet, M.C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shukla, P.; Ayyachamy, M.; Permaul, K.; Singh, S. Improved bioethanol production through simultaneous saccharification and fermentation of lignocellulosic agricultural wastes by Kluyveromyces marxianus 6556. World J. Microbiol. Biotechnol. 2010, 26, 1041–1046. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hahn-Hagerdal, B.; Liden, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Patsalou, M.; Menikea, K.K.; Makri, E.; Vasquez, M.I.; Drouza, C.; Koutinas, M. Development of a citrus peel-based biorefinery strategy for the production of succinic acid. J. Clean. Prod. 2017, 166, 706–716. [Google Scholar] [CrossRef]
- Talebnia, F.; Pourbafrani, M.; Lundin, M.; Taherzadeh, M.J. Optimization study of citrus wastes saccharification by dilute-acid hydrolysis. Bioresources 2008, 3, 108–122. [Google Scholar]
- Pourbafrani, M.; Forgacs, G.; Horvath, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of biofuels, limonene and pectin from citrus wastes. Bioresour. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; Garcia-Vidal, L.; Gonzalez-Castaneda, F.D.P.; Lopez-Gomez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef]
- Lennartsson, P.R.; Ylitervo, P.; Larsson, C.; Edebo, L.; Taherzadeh, M.J. Growth tolerance of Zygomycetes Mucor indicus in orange peel hydrolysate without detoxification. Process Biochem. 2012, 47, 836–842. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Rozes, N.; Guillamon, J.M. Effect of the nitrogen source on the fatty acid composition of Saccharomyces cerevisiae. Food Microbiol. 2003, 20, 255–258. [Google Scholar] [CrossRef]
- Seong, Y.J.; Lee, H.J.; Lee, J.E.; Kim, S.; Lee, D.Y.; Kim, K.H.; Park, Y.C. Physiological and metabolomic analysis of Issatchenkia orientalis MTY1 with multiple tolerance for cellulosic bioethanol production. Biotechnol. J. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koppar, A.; Pullammanappallil, P. Anaerobic digestion of peel waste and wastewater for on site energy generation in a citrus processing facility. Energy 2013, 60, 62–68. [Google Scholar] [CrossRef]
- Sanjaya, A.P.; Cahyanto, M.N.; Millati, R. Mesophilic batch anaerobic digestion from fruit fragments. Renew. Energy 2016, 98, 135–141. [Google Scholar] [CrossRef]
- Calabro, P.S.; Pontoni, L.; Porqueddu, I.; Greco, R.; Pirozzi, F.; Malpei, F. Effect of the concentration of essential oil on orange peel waste biomethanization: Preliminary batch results. Waste Manag. 2016, 48, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lotito, A.M.; Sanctis, M.D.; Pastore, C.; Iaconi, C.D. Biomethanization of citrus waste: Effect of waste characteristics and of storage on treatability and evaluation of limonene degradation. J. Environ. Manag. 2018, 215, 366–376. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef]
- Faravash, R.S.; Ashtiani, F.Z. The effect of pH, ethanol volume and acid washing time on the yield of pectin extraction from peach pomace. Int. J. Food Sci. Technol. 2007, 42, 1177–1187. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Cameron, R.G.; Grohmann, K. Effect of seasonal variation on enzymatic hydrolysis of valencia orange peel waste. Proc. Fla. State Hortic. Soc. 2005, 118, 419–422. [Google Scholar]
- Koutinas, M.; Patsalou, M.; Stavrinou, S.; Vyrides, I. High temperature alcoholic fermentation of orange peel by the newly isolated thermotolerant Pichia kudriavzevii KVMP10. Lett. Appl. Microbiol. 2015, 62, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, Ameican Water Works Association, Water Environment Federation: Denver, CO, USA, 2012; p. 541. [Google Scholar]
- Vardanyan, A.; Kafa, N.; Konstantinidis, V.; Shin, S.G.; Vyrides, I. Phosphorus dissolution from dewatered anaerobic sludge: Effect of pHs, microorganisms, and sequential extraction. Bioresour. Technol. 2018, 249, 464–472. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


 ), dried citrus peel waste (DCPW) (
), dried citrus peel waste (DCPW) (  ), and solid biorefinery residues (BR) (
), and solid biorefinery residues (BR) (  ). A control fermentation was conducted without the addition of CPW (
). A control fermentation was conducted without the addition of CPW (  ), while all experiments were conducted at 37 °C. Dashed lines represent the time where substrate refeed was applied.
), while all experiments were conducted at 37 °C. Dashed lines represent the time where substrate refeed was applied.
 ), dried citrus peel waste (DCPW) (
), dried citrus peel waste (DCPW) (  ), and solid biorefinery residues (BR) (
), and solid biorefinery residues (BR) (  ). A control fermentation was conducted without the addition of CPW (
). A control fermentation was conducted without the addition of CPW (  ), while all experiments were conducted at 37 °C. Dashed lines represent the time where substrate refeed was applied.
), while all experiments were conducted at 37 °C. Dashed lines represent the time where substrate refeed was applied.
 ), DCPW (
), DCPW (  ), solid BR (
), solid BR (  ), and in the control experiment (
), and in the control experiment (  ).
).
 ), DCPW (
), DCPW (  ), solid BR (
), solid BR (  ), and in the control experiment (
), and in the control experiment (  ).
).

 ), 6 g L−1 (
), 6 g L−1 (  ), 12 g L−1 (
), 12 g L−1 (  ), and 24 g L−1 (
), and 24 g L−1 (  ) initial volatile solids of CPW. A control fermentation was performed without the addition of CPW (
) initial volatile solids of CPW. A control fermentation was performed without the addition of CPW (  ), while all experiments were conducted at 37 °C.
), while all experiments were conducted at 37 °C.
 ), 6 g L−1 (
), 6 g L−1 (  ), 12 g L−1 (
), 12 g L−1 (  ), and 24 g L−1 (
), and 24 g L−1 (  ) initial volatile solids of CPW. A control fermentation was performed without the addition of CPW (
) initial volatile solids of CPW. A control fermentation was performed without the addition of CPW (  ), while all experiments were conducted at 37 °C.
), while all experiments were conducted at 37 °C.
| Experiment | Reducing Sugars Consumed (g L−1) | Final Ethanol Titre (g L−1) |
|---|---|---|
| P. kudriavzevii KVMP10 | ||
| 108 °C, 10 min | 11.0 | 4.7 |
| 108 °C, 20 min | 17.9 | 5.2 |
| 116 °C, 10 min | 12.1 | 5.8 |
| 116 °C, 20 min | 30.9 | 2.9 |
| 125 °C, 10 min | 15.5 | 5.8 |
| 125 °C, 20 min | 20.5 | 6.7 |
| K. marxianus | ||
| 108 °C, 10 min | 9.8 | 3.6 |
| 108 °C, 20 min | 3.1 | 1.6 |
| 116 °C, 10 min | 17.2 | 4.6 |
| 116 °C, 20 min | 10.9 | 2.3 |
| 125 °C, 10 min | 7.5 | 3.8 |
| 125 °C, 20 min | 18.6 | 1.8 |
| S. cerevisiae | ||
| 108 °C, 10 min | 6.9 | 3.5 |
| 108 °C, 20 min | 5.4 | 1.8 |
| 116 °C, 10 min | 8.6 | 4.2 |
| 116 °C, 20 min | 6.0 | 2.7 |
| 125 °C, 10 min | 8.1 | 2.2 |
| 125 °C, 20 min | 5.9 | 3.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A Citrus Peel Waste Biorefinery for Ethanol and Methane Production. Molecules 2019, 24, 2451. https://doi.org/10.3390/molecules24132451
Patsalou M, Samanides CG, Protopapa E, Stavrinou S, Vyrides I, Koutinas M. A Citrus Peel Waste Biorefinery for Ethanol and Methane Production. Molecules. 2019; 24(13):2451. https://doi.org/10.3390/molecules24132451
Chicago/Turabian StylePatsalou, Maria, Charis G. Samanides, Eleni Protopapa, Stella Stavrinou, Ioannis Vyrides, and Michalis Koutinas. 2019. "A Citrus Peel Waste Biorefinery for Ethanol and Methane Production" Molecules 24, no. 13: 2451. https://doi.org/10.3390/molecules24132451