Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase
Abstract
:1. Introduction
2. Results
Synthesis of Compounds 5a–r
3. Discussion
3.1. In Vitro Biological Activity Evaluation
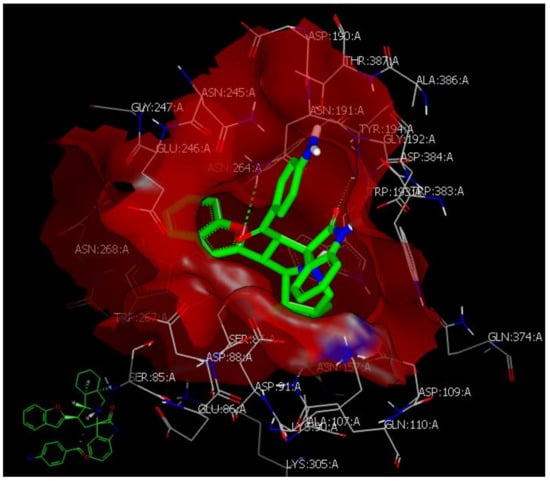
3.2. Docking Study
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of Chalcones 2a–f (GP1)
4.3. General Procedure for the Synthesis of Compounds 5a–r (GP2)
4.4. Protocols for the α-Glucosidase Inhibition and α-Amylase Assays
4.4.1. Reagents
4.4.2. α-Glucosidase Inhibition Assay
4.4.3. α-Amylase Assay
4.4.4. Calculation of Inhibition Efficiency
4.5. Docking Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus; WHO/NCD/NCS/99.2; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- IDF. Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2014. [Google Scholar]
- Guariguata, L.; Whiting, D.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Marcovecchio, M.; Mohn, A.; Chiarelli, F. Type 2 diabetes mellitus in children and adolescents. J. Endocrinol. Investig. 2005, 28, 853–863. [Google Scholar] [CrossRef]
- Najafian, M.; Ebrahim-Habibi, A.; Hezareh, N.; Yaghmaei, P.; Parivar, K.; Larijani, B. Trans-Chalcone: A novel small molecule inhibitor of mammalian alpha-amylase. J. Mol. Boil. Rep. 2010, 38, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhair, S.; Dowaidar, A.; Kamal, H. Inhibitory effect of dates extracts on α-amylase and α-glucosidase enzymes relevant to non-insulin dependent diabetes mellitus. J. Biochem. Technol. 2010, 2, 158–160. [Google Scholar]
- Afonne, O.J.; Orisakwe, O.E.; Obi, E.; Orish, C.; Akumka, D.D. Some pharmacological properties of Synclisia scabrida III. Indian J. Pharmacol. 2000, 32, 239–241. [Google Scholar]
- Daisy, P.; Jasmine, R.; Ignacimuthu, S.; Murugan, E. A novel steroid from Elephantopus scaber L. an ethnomedicinal plant with antidiabetic activity. J. Phytomed. 2009, 16, 252–257. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocinnicotinamide-induced type 2 diabetic rats. J. Ethnopharmacol. 2005, 97, 369–374. [Google Scholar] [CrossRef]
- Akerele, O. Traditional Medicine: Nature’s medicinal bounty; don’t throw it away. World Health Forum 1993, 14, 390–395. [Google Scholar]
- Geethalakshmi, R.; Sarada, D.V.L.; Marimuthu, P.; Ramasamy, K. A-amylase inhibitory activity of Trianthema decandra L. Int. J. Biotechnol. Biochem. 2010, 6, 369–376. [Google Scholar]
- Dewanjee, S.; Das, A.K.; Sahu, R.; Gangopadhyay, M. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem. Toxicol. 2009, 47, 2679–2685. [Google Scholar] [CrossRef]
- Manna, K.; Agrawal, Y.K. Design, synthesis, and antitubercular evaluation of novel series of 3-benzofuran-5-aryl-1-pyrazolyl-pyridylmethanone and 3-benzofuran-5-aryl-1-pyrazolylcarbonyl-4-oxo-naphthyridin analogs. Eur. J. Med. Chem. 2010, 45, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel tacrine–benzofuran hybrids as potent multitarget-directed ligands for the treatment of Alzheimer’s disease: Design, synthesis, biological evaluation, and X-ray crystallography. J. Med. Chem. 2015, 59, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, P.; Tewari, A.K. Design, synthesis, docking and anti-inflammatory evaluation of novel series of benzofuran based prodrugs. Bioorg. Med. Chem. Lett. 2014, 24, 2251–2255. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Patil, M.R.; Chethana, K.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Rida, S.M.; El-Hawash, S.A.; Fahmy, H.T.; Hazza, A.A.; El-Meligy, M.M. Synthesis and in vitro evaluation of some novel benzofuran derivatives as potential anti-HIV-1, anticancer, and antimicrobial agents. Arch. Pharm. Res. 2006, 29, 16. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.M.; Amin, K.M.; Ali, H.I.; Abdalla, M.M.; Ahmed, E.Y. Design, synthesis and anticancer activity of benzofuran derivatives targeting VEGFR-2 tyrosine kinase. RSC Adv. 2014, 4, 11569–11579. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Modukuri, R.K.; Sonkar, R.; Rao, K.B.; Bhatia, G. Hybrid benzofuran–bisindole derivatives: New prototypes with promising anti-hyperlipidemic activities. Eur. J. Med. Chem. 2013, 68, 38–46. [Google Scholar] [CrossRef]
- Bhovi, V.K.; Bodke, Y.D.; Biradar, S.; Swamy, B.K.; Umesh, S. A facile synthesis of bromo-substituted benzofuran containing thiazolidinone nucleus bridged with quinoline derivatives: Potent analgesic and antimicrobial agents. Phosphorus Sulfur Silicon 2009, 185, 110–116. [Google Scholar] [CrossRef]
- Dawood, K.M.; Abdel-Gawad, H.; Rageb, E.A.; Ellithey, M.; Mohamed, H.A. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 2006, 14, 3672–3680. [Google Scholar] [CrossRef]
- Ashwood, V.A.; Field, M.J.; Horwell, D.C.; Julien-Larose, C.; Lewthwaite, R.A.; McCleary, S.; Pritchard, M.C.; Raphy, J.; Singh, L. Utilization of an intramolecular hydrogen bond to increase the CNS penetration of an NK1 receptor antagonist. J. Med. Chem. 2001, 44, 2276–2285. [Google Scholar] [CrossRef]
- He, Y.; Zeng, L.F.; Yu, Z.H.; He, R.; Liu, S.; Zhang, Z.Y. Bicyclic benzofuran and indole-based salicylic acids as protein tyrosine phosphatase inhibitors. Bioorg. Med. Chem. 2012, 20, 1940–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.S.; Kumar, D.; Bodduri, V.V.; Tarani, P.S.; Zhao, B.X.; Miao, J.Y.; Jang, K.; Shin, D.S. Microwave-assisted parallel synthesis of benzofuran-2-carboxamide derivatives bearing anti-inflammatory, analgesic and antipyretic agents. Tetrahedron Lett. 2014, 55, 2796–2800. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.Y.; Zhou, J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016, 2, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.A. The Ganges. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Semmelhack, M.F., Eds.; Pergmon Press: Oxford, UK, 1991; Volume 4, p. 1111. [Google Scholar]
- Sun, Y.; Liu, J.; Sun, T.; Zhang, X.; Yao, J.; Kai, M.; Jiang, X.; Wang, R. Anti-cancer small molecule JP-8g exhibits potent in vivo anti-inflammatory activity. Sci. Rep. 2014, 4, 4372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, J.; Jiang, X.; Sun, T.; Liu, L.; Zhang, X.; Ding, S.; Li, J.; Zhuang, Y.; Wang, Y.; et al. One-step synthesis of chiral oxindole-type analogues with potent anti-inflammatory and analgesic activities. Sci. Rep. 2015, 5, 13699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Cao, Y.; Wang, Y.; Liu, L.; Shen, F.; Wang, R. A unique approach to the concise synthesis of highly optically active spirooxazolines and the discovery of a more potent oxindole-type phytoalexin analogue. J. Am. Chem. Soc. 2010, 132, 15328–15333. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2−p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Substituted Spirooxindoles. U.S. Patent 9,822,128, 21 November 2017. [Google Scholar]
- Islam, M.S.; Ghawas, H.M.; El-Senduny, F.F.; Al-Majid, A.M.; Elshaier, Y.A.; Badria, F.A.; Barakat, A. Synthesis of new thiazolo-pyrrolidine–(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.; Ghabbour, H.A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. [Google Scholar] [CrossRef] [Green Version]
- Lotfy, G.; El Sayed, H.; Said, M.M.; Aziz, Y.M.A.; Al-Dhfyan, A.; Al-Majid, A.M.; Barakat, A. Regio-and stereoselective synthesis of new spirooxindoles via 1, 3-dipolar cycloaddition reaction: Anticancer and molecular docking studies. J. Photochem. Photobiol. B Biol. 2018, 180, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, G.; Said, M.M.; El Sayed, H.; El Sayed, H.; Al-Dhfyan, A.; Aziz, Y.M.A.; Barakat, A. Synthesis of new spirooxindole-pyrrolothiazole derivatives: Anti-cancer activity and molecular docking. Bioorg. Med. Chem. 2017, 25, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Clinical efficacy of acarbose in diabetes mellitus: A critical review of controlled trials. Diabetes Metab. 1998, 24, 311–320. [Google Scholar] [PubMed]
- Islam, M.S.; Barakat, A.; Al-Majid, A.M.; Ali, M.; Yousuf, S.; Choudhary, M.I.; Khalil, R.; Ul-Haq, Z. Catalytic asymmetric synthesis of indole derivatives as novel α-glucosidase inhibitors in vitro. Bioorg. Chem. 2018, 79, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Elshaier, Y.A.; Shaaban, M.A.; El Hamid, M.K.A.; Abdelrahman, M.H.; Abou-Salim, M.A.; Elgazwi, S.M.; Halaweish, F. Design and synthesis of pyrazolo [3,4-d] pyrimidines: Nitric oxide releasing compounds targeting hepatocellular carcinoma. Bioorg. Med. Chem. 2017, 25, 2956–2970. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Orlovsky, N.I.; Foley, M.H.; Kwiatkowski, K.J.; Bahr, C.M.; Maynard, M.; Demeler, B.; Koropatkin, N.M. Molecular details of a starch utilization pathway in the human gut symbiont E ubacterium rectale. Mol. Microbiol. 2015, 95, 209–230. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5a–r are available from the authors. |





| # | Compound | α-Amylase | α-Glucosidase | α-Amylase Selectivity b | α-Glucosidase Selectivity c |
|---|---|---|---|---|---|
| IC50 (μM ± SD) a | |||||
| 1 |  5a 5a | 693.22 ± 0.10 | 465.12 ± 0.12 | 0.67 | 1.49 |
| 2 |  5b 5b | 747.08 ± 0.34 | 545.01 ± 1.09 | 0.72 | 1.37 |
| 3 |  5c 5c | 718.00 ± 0.27 | 585.11 ± 0.02 | 0.81 | 1.22 |
| 4 |  5d 5d | 728.13 ± 0.28 | 549.17 ± 1.06 | 0.75 | 1.32 |
| 5 |  5e 5e | 710.07 ± 0.10 | 554.12 ± 1.42 | 0.78 | 1.28 |
| 6 |  5f 5f | 670.14 ± 0.10 | 534.04 ± 1.09 | 0.79 | 1.25 |
| 7 |  5g 5g | 690.09 ± 0.06 | 554.12 ± 1.42 | 0.80 | 1.24 |
| 8 |  5h 5h | 589.04 ± 0.25 | 494.10 ± 0.04 | 0.83 | 1.19 |
| 9 |  5i 5i | 779.08 ± 0.51 | 684.12 ± 0.35 | 0.87 | 1.13 |
| 10 |  5j 5j | 39.02 ± 1.73 | 29.20 ± 0.33 | 0.74 | 1.33 |
| 11 |  5k 5k | 49.28 ± 1.09 | 39.10 ± 0.54 | 0.79 | 1.26 |
| 12 |  5l 5l | 558.07 ± 0.18 | 414.12 ± 0.52 | 0.74 | 1.37 |
| 13 |  5m 5m | 95.26 ± 0.27 | 69.11 ± 0.34 | 0.72 | 1.37 |
| 14 |  5n 5n | 185.23 ± 1.06 | 98.23 ± 1.24 | 0.53 | 1.88 |
| 15 |  5o 5o | 115.42 ± 0.07 | 68.18 ± 1.54 | 0.59 | 1.69 |
| 16 |  5p 5p | 488.02 ± 1.11 | 392.13 ± 1.07 | 0.80 | 1.24 |
| 17 |  5q 5q | 37.22 ± 1.49 | 26.29 ± 0.45 | 0.70 | 1.41 |
| 18 |  5r 5r | 22.61 ± 0.54 | 14.05 ± 1.03 | 0.62 | 1.60 |
| STD | Acarbose (μM) | 0.75 + 0.07 | 2.35 + 0.13 | 3.13 | 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase. Molecules 2019, 24, 2342. https://doi.org/10.3390/molecules24122342
Altowyan MS, Barakat A, Al-Majid AM, Al-Ghulikah HA. Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase. Molecules. 2019; 24(12):2342. https://doi.org/10.3390/molecules24122342
Chicago/Turabian StyleAltowyan, Mezna Saleh, Assem Barakat, Abdullah Mohammed Al-Majid, and H.A. Al-Ghulikah. 2019. "Spiroindolone Analogues as Potential Hypoglycemic with Dual Inhibitory Activity on α-Amylase and α-Glucosidase" Molecules 24, no. 12: 2342. https://doi.org/10.3390/molecules24122342