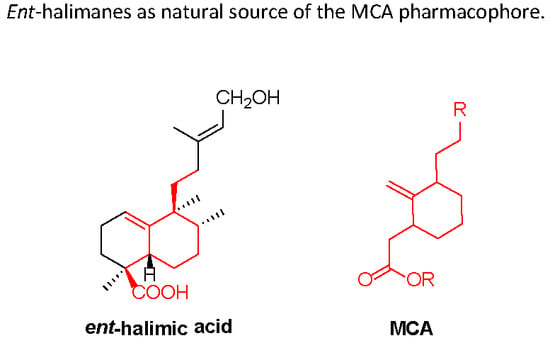
The Methylene-Cycloalkylacetate (MCA) Scaffold in Terpenyl Compounds with Potential Pharmacological Activities
Abstract
:1. Halimanes Containing the MCA Fragment
1.1. Group I: The Halim-1(10)-Enes Group
1.2. Group II: The Halim-5-Enes Group
1.3. Group III: Secohalimanes and 4-Enes Derivatives
2. Ent-Halimic Acid as a Precursor of Biologically Active Compounds and Other Derivatives of Interest
3. Synthetic Transformations of Ent-halimic Acid Methyl Ester, 2
3.1. Synthesis of Ent-Halimanolides
3.2. Chettaphanin Synthesis
3.3. Sesterterpenolide Synthesis
3.4. Synthesis of Hybrid Compounds of Sesterterpenolides with Glycerols
3.5. Synthesis of Rearranged Compounds
3.5.1. Synthesis of Ent-Labdanes from Ent-Halimanes.
3.5.2. Synthesis of Abeopicrasanes from ent-Halimanes.
3.5.3. Synthesis of [4.3.3] Propellanes from Ent-Halimanes.
3.6. Quinone/Hydroquinone Sesquiterpenes
3.7. Sesqui- and Diterpene-Alkaloids
3.7.1. Synthesis of Diterpene-alkaloid, (+)-agelasine C [7]
3.7.2. Synthesis of the Indole Diterpene-alkaloid (+)-thiersindole C
3.7.3. Synthesis of Sesquiterpenil-Indoles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lankri, D.; Haham, D.; Lahiani, A.; Lazarovici, P.; Tsvelikhovsky, D. Methylene-cycloalkylacetate (MCA) scaffold-based compounds as novel neurotropic agents. ACS Chem. Neurosci. 2018, 9, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Marcos, I.S.; Pedrero, A.B.; Sexmero, M.J.; Diez, D.; García, N.; Escola, M.A.; Basabe, P.; Conde, A.; Moro, R.F.; Urones, J.G. Synthesis of ent-Halimanolides from ent-Halimic Acid. Synthesis 2005, 19, 3301–3310. [Google Scholar] [CrossRef]
- Miyaoka, H.; Yamanishi, M.; Kajiwara, Y.; Yamada, Y. Total synthesis of cladocorans A and B: A structural revision. J. Nat. Prod. 2003, 68, 3476–3479. [Google Scholar]
- Marcos, I.S.; Hernández, F.A.; Sexmero, M.J.; Díez, D.; Basabe, P.; Pedrero, A.B.; García, N.; Sanz, F.; Urones, J.G. Synthesis and absolute configuration of (−)-chettaphanin II. Tetrahedron Lett. 2002, 43, 1243–1245. [Google Scholar] [CrossRef]
- Marcos, I.S.; Hernández, F.A.; Sexmero, M.J.; Díez, D.; Basabe, P.; Pedrero, A.B.; García, N.; Urones, J.G. Synthesis and absolute configuration of (−)-chettaphanin I and (−)-chettaphanin II. Tetrahedron 2003, 59, 685–694. [Google Scholar] [CrossRef]
- Marcos, I.S.; Pedrero, A.B.; Sexmero, M.J.; Diez, D.; Basabe, P.; Hernández, F.A.; Broughton, H.B.; Urones, J.G. Synthesis and absolute configuration of the supposed structure of cladocoran A and B. Synlett 2002, 01, 0105–0109. [Google Scholar] [CrossRef]
- Marcos, I.S.; García, N.; Sexmero, M.J.; Basabe, P.; Díez, D.; Urones, J.G. Synthesis of (+)-agelasine C. A structural revision. Tetrahedron 2005, 61, 11672–11678. [Google Scholar] [CrossRef]
- Marcos, I.S.; Escola, M.A.; Moro, R.F.; Basabe, P.; Diez, D.; Mollinedo, F.; Urones, J.G. Synthesis of (+)-thiersindole, C. Synlett. 2007, 13, 2017–2022. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Nagahori, H.; Ogiso, A.; Mishima, H. Chettaphanin-I, a novel furanoditerpenoid. Tetrahedron Lett. 1970, 11, 1095–1098. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Ogiso, A.; Mishima, H. Chettaphanin-II, a novel furanoditerpenoid. Tetrahedron Lett. 1971, 12, 839–842. [Google Scholar] [CrossRef]
- Wang, G.-C.; Li, J.-G.; Li, G.-Q.; Xu, J.-J.; Wu, X.; Ye, W.-C.; Li, Y.-L. Clerodane diterpenoids from Croton crassifolius. J. Nat. Prod. 2012, 75, 2188–2192. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, H.-H.; Qi, F.-M.; Xiong, H.-Y.; Dong, L.-L.; Fan, G.-X.; Fei, D.-Q. A New halimane diterpenoid from Croton crassifolius. Bull. Korean Chem. Soc. 2014, 35, 1556–1558. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Ruangrungsi, N.; Watanabe, T.; Kawahata, M.; Therrien, B.; Yamaguchi, K.; Ishikawa, T. ent-Halimane diterpenes and a guaiane sesquiterpene from Cladogynos orientalis. J. Nat. Prod. 2005, 68, 7–10. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Basabe, P.; Sexmero, M.J.; Carrillo, H.; Melchor, M.J. Minor diterpenoids from Halimium viscosum. Phytochemistry 1994, 37, 1359–1361. [Google Scholar] [CrossRef]
- Urones, J.G.; De Pascual Teresa, J.; Marcos, I.S.; Díez Martín, D.; Martín Garrido, N.; Guerra, R.A. Diterpenoids from Halimium viscosum. Phytochemistry 1987, 26, 1077–1079. [Google Scholar] [CrossRef]
- Khoo, S.F.; Oehlschlager, A.C.; Ourisson, G. Structure and stereochemistry of the diterpenes of Hymenaea courbaril (Caesalpinioideae) seed pod resin. Tetrahedron 1973, 29, 3379–3388. [Google Scholar] [CrossRef]
- Urones, J.G.; Sexmero, M.J.; Hernández, F.A.; Basabe, P.; Pedrero, A.B.; Diez, D.; Marcos, I.S. Minor Diterpenoids from Halimium viscosum. Nat. Prod. Lett. 2001, 15, 387–391. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Sexmero, M.J.; Basabe, P.; Lithgow, A.M. ent-Halimane diterpenes from Halimium viscosum. Phytochemistry 1990, 29, 1247–1251. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; Urones, J.G.; Carrillo, H. Componentes del Halimium umbellatum (L.) Spach III. Ácido halímico [ent-9,1-friedolabd-1(10),13-dien-15-hidroxi-18-oico] y derivados. An. Quim. 1978, 74, 488–493. [Google Scholar]
- Abdel-Kader, M.; Berger, J.M.; Slebodnick, C.; Hoch, J.; Malone, S.; Wisse, J.H.; Werkhoven, M.C.M.; Mamber, S.; Kingston, D.G.I. Isolation and absolute configuration of ent-Halimane diterpenoids from Hymenaea courbaril from the suriname rain forest. J. Nat. Prod. 2002, 65, 11–15. [Google Scholar] [CrossRef]
- Rodilla, J.M.L.; Mendonça, D.I.D.; Ismael, M.I.G.; Figueiredo, J.A.; Silva, M.L.A.; Lopes, E. BI- and Tricyclic Diterpenoids from Halimium viscosum. Nat. Prod. Lett. 2001, 15, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-Q.; Tang, S.; Song, W.-B.; Wang, W.-Q.; Huang, M.; Xuan, L.-J. Crassins A–H, Diterpenoids from the roots of Croton crassifolius. J. Nat. Prod. 2017, 80, 254–260. [Google Scholar] [CrossRef] [PubMed]
- De Pascual Teresa, J.; Urones, J.G.; Basabe, P.; Carrillo, H.; Muñoz, M.A.G.; Marcos, I.S. Diterpenoids of Halimium viscosum. Phytochemistry 1985, 24, 791–794. [Google Scholar] [CrossRef]
- De Mendonça, D.I.M.D.; Rodilla, J.M.L.; Lithgow, A.M.; Marcos, I.S. Hydrohalimic acids from Halimium viscosum. Phytochemistry 1997, 44, 1301–1307. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; Urones, J.G.; Carrillo, H.; Muñoz, M.A.G. Ácidos diterpénicos del Halimium viscosum (Wilk) P. Silva. Ácido hidrohalímico (ácido ent-9,1-friedolabd-1(10)-en-15-hidroxi-18-oico) y derivados. An. Quim. 1979, 75, 140–143. [Google Scholar]
- Rodilla, J.M.L.; De Mendonça, D.I.M.; Urones, J.G.; Moro, R.F. Hydroxylated diterpenoids from Halimium viscosum. Phytochemistry 1998, 49, 817–822. [Google Scholar] [CrossRef]
- Monteiro, A.F.; Batista, J.M.; Machado, M.A.; Severino, R.P.; Blanch, E.W.; Bolzani, V.S.; Vieira, P.C.; Severino, V.G.P. Structure and absolute configuration of diterpenoids from Hymenaea stigonocarpa. J. Nat. Prod. 2015, 78, 1451–1455. [Google Scholar] [CrossRef]
- Jakupovic, J.; Ellmauerer, E.; Bohlmann, F.; Whittemori, A.; Gage, D. Diterpenes from Eupatorium turbinatum. Phytochemistry 1986, 25, 2677–2680. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Sexmero, M.J.; Basabe, P.; Lithgow, A.M. Nor-ent-halimanes from Halimium viscosum. Phytochemistry 1990, 29, 3597–3600. [Google Scholar] [CrossRef]
- Pinto, Â.C.; Antunes, O.A.C.; Pizzolatti, M.G.; Rumjanek, V.M. A tetranorfriedolabdane diterpene from Vellozia stipitata. Phytochemistry 1996, 42, 771–774. [Google Scholar] [CrossRef]
- Rijo, P.; Gaspar-Marques, C.; Simões, M.F.; Jimeno, M.L.; Rodríguez, B. Further diterpenoids from Plectranthus ornatus and P. grandidentatus. Biochem. Syst. Ecol. 2007, 35, 215–223. [Google Scholar] [CrossRef]
- Rijo, P.; Rodriguez, B.; Duarte, A.; Simoes, M.F. Antimicrobial properties of Plectranthus ornatus extracts, 11-acetoxyhalima-5, 13-dien-15-oic acid metabolite and its derivatives. Natur. Products J. 2011, 1, 57–64. [Google Scholar]
- Jakupovic, J.; Baruah, R.N.; Zdero, C.; Eid, F.; Pathak, V.P.; Chau-thi, T.V.; Bohlmann, F.; King, R.M.; Robinson, H. Further diterpenes from plants of the compositae, subtribe solidagininae. Phytochemistry 1986, 25, 1873–1881. [Google Scholar] [CrossRef]
- Tsichritzis, F.; Jakupovic, J. Diterpenes and other constituents from Relhania species. Phytochemistry 1990, 29, 3173–3187. [Google Scholar] [CrossRef]
- Del Corral, S.; Cuffini, S.L.; Cardoso, S.G.; Bortoluzzid, A.J.; Palacios, S.M. Phytotoxic halimanes isolated from Baccharis salicifolia (Ruiz & Pad.) Pers. Phytochem. Lett. 2012, 5, 280–282. [Google Scholar]
- Aldhaher, A.; Langat, M.; Ndunda, B.; Chirchir, D.; Midiwo, J.O.; Njue, A.; Schwikkard, S.; Carew, M.; Mulholland, D. Diterpenoids from the roots of Croton dichogamus Pax. Phytochemistry 2017, 144, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Banerjee, S.; Bohlmann, F.; King, R.M.; Robinson, H. New diterpenes from Chiliotrichium rosmarinifolium and Nardophyllum lanatum. Tetrahedron 1986, 42, 1305–1313. [Google Scholar] [CrossRef]
- Sánchez, M.; Mazzuca, M.; Veloso, M.J.; Fernández, L.R.; Siless, G.; Puricelli, L.; Palermo, J.A. Cytotoxic terpenoids from Nardophyllum bryoides. Phytochemistry 2010, 71, 1395–1399. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; D’Angelo, G.; Gallicchio, M.; Chambery, A.; Monaco, P. Structure elucidation and hepatotoxicity evaluation against HepG2 human cells of neo-clerodane diterpenes from Teucrium polium L. Phytochemistry 2011, 72, 2037–2044. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.-H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Bohlmann, F.; Abraham, W.-R.; King, R.M.; Robinson, H. Diterpenes from Koanophyllon species. Phytochemistry 1981, 20, 1903–1906. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; Niemeyer, H.M. Friedolabdanes and other constituents from chilean Haplopappus species. Phytochemistry 1991, 30, 3669–3677. [Google Scholar] [CrossRef]
- Siems, K.; Jakupovic, J.; Castro, V.; Poveda, L. Constituents of two Acalypha species. Phytochemistry 1996, 41, 851–853. [Google Scholar] [CrossRef]
- Du, K.; De Mieri, M.; Neuburger, M.; Zietsman, P.C.; Marston, A.; van Vuuren, S.F.; Ferreira, D.; Hamburger, M.; van der Westhuizen, J.H. Labdane and clerodane diterpenoids from Colophospermum mopane. J. Nat. Prod. 2015, 78, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Adnani, N.; Braun, D.R.; Ellis, G.A.; Barns, K.J.; Parker-Nance, S.; Guzei, I.A.; Bugni, T.S. Micromonohalimanes A and B: Antibacterial halimane-Type diterpenoids from a marine micromonospora species. J. Nat. Prod. 2016, 79, 2968–2972. [Google Scholar] [CrossRef]
- Ono, M.; Yamasaki, T.; Konoshita, M.; Ikeda, T.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Five New Diterpenoids, viteagnusins A—E, from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2008, 56, 1621–1624. [Google Scholar] [CrossRef]
- Wang, W.-q.; Yin, Y.-p.; Jun, L.; Xuan, L.-j. Halimane-type diterpenoids from Vitex rotundifolia and their anti-hyperlipidemia activities. Phytochemistry 2018, 146, 56–62. [Google Scholar] [CrossRef]
- Pinto, A.C.; Antunes, O.A.C.; Rezende, C.M.; Correia, C.R.D. Minor cleisthantane and tetranorfriedolabdane from Vellozia flavicans. Phytochemistry 1995, 38, 1269–1271. [Google Scholar] [CrossRef]
- Kihampa, C.; Nkunya, M.H.H.; Joseph, C.C.; Magesa, S.M.; Hassanali, A.; Heydenreich, M.; Kleinpeter, E. Anti-mosquito and antimicrobial nor-halimanoids, isocoumarins and an anilinoid from Tessmannia densiflora. Phytochemistry 2009, 70, 1233–1238. [Google Scholar] [CrossRef]
- Nyandoro, S.S. Some rare Tanzanian plant species as sources of less common metabolites: Biomedical potential and conservation status. J. Pharmacogn. Phytochem. 2014, 3, 147–157. [Google Scholar]
- Kawashima, T.; Nakatsu, T.; Fukazawa, Y.; Itô, S. Diterpenic lactones of Mallotus repandus. Heterocycles 1976, 5, 227–232. [Google Scholar]
- Fernández-Gadea, F.; Pascual, C.; Rodríguez, B.; Savona, G. 6-epiteucrin A, a neo-clerodane diterpenoid from Teucrium chamaedrys. Phytochemistry 1983, 22, 723–725. [Google Scholar] [CrossRef]
- Fujita, E.; Uchida, I.; Fujita, T.; Masaki, N.; Osaki, K. Teucvin, a novel furanoid norditerpene from Teucrium viscidum var. Miquelianum. Chem. Commun. 1973, 793–794. [Google Scholar] [CrossRef]
- Fujita, E.; Uchida, I.; Fujita, T. Terpenoids. Part XXXII. Structure and stereochemistry of teucvin, a novel norclerodane-type diterpene from Teucrium viscidum var. Miquelianum. Perkin Trans. 1 1974, 1547–1555. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Foubert, K.; Chacha, M.; Kapingu, M.C.; Magadula, J.J.; Moshi, M.M.; Lemière, F.; Goubitz, K.; Fraanje, J.; Peschar, R.; Vlietinck, A.; Apers, S.; Pieters, L. New furanoditerpenoids from Croton jatrophoides. Planta Med. 2009, 75, 262–264. [Google Scholar] [CrossRef]
- Marcos, I.S.; Pedrero, A.B.; Sexmero, M.J.; Diez, D.; Basabe, P.; Hernández, F.A.; Urones, J.G. Synthesis and absolute configuration of three natural ent-halimanolides with biological activity. Tetrahedron Lett. 2003, 44, 369–372. [Google Scholar] [CrossRef]
- Marcos, I.S.; Conde, A.; Moro, R.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J. Synthesis of an ent-Halimanolide from ent-Halimic Acid. Molecules 2008, 13, 1120–1134. [Google Scholar] [CrossRef]
- Marcos, I.S.; Gonzalez, J.L.; Sexmero, M.J.; Díez, D.; Basabe, P.; Williams, D.J.; Simmonds, M.S.J.; Urones, J.G. Diterpenic α- and β-hydroxybutanolides with antifeedant activity: Semisynthesis and absolute configuration. Tetrahedron Lett. 2000, 41, 2553–2557. [Google Scholar] [CrossRef]
- Marcos, I.S.; Escola, M.A.; Moro, R.F.; Basabe, P.; Diez, D.; Sanz, F.; Mollinedo, F.; de la Iglesia-Vicente, J.; Sierra, B.G.; Urones, J.G. Synthesis of novel antitumoral analogues of dysidiolide from ent-halimic acid. Bioorg. Med. Chem. 2007, 15, 5719–5737. [Google Scholar] [CrossRef]
- Marcos, I.S.; Pedrero, A.B.; Sexmero, M.J.; Diez, D.; Basabe, P.; García, N.; Moro, R.F.; Broughton, H.B.; Mollinedo, F.; Urones, J.G. Synthesis of Bioactive Sesterterpenolides from ent-Halimic Acid. 15-Epi-ent-cladocoran A and B. J. Nat. Prod. 2003, 68, 7496–7504. [Google Scholar]
- Gil-Mesón, A.; Roncero, A.; Tobal, I.; Basabe, P.; Díez, D.; Mollinedo, F.; Marcos, I.S. Synthesis of bioconjugate sesterterpenoids with phospholipids and polyunsaturated fatty acids. Molecules 2016, 21, 47. [Google Scholar] [CrossRef]
- Marcos, I.S.; Sexmero, M.; Hernández, F.; Corrales, M.; Basabe, P.; Díez, D.; Urones, J.G. Synthetic studies towards the ent-Labdane diterpenoids: rearrangement of ent-Halimanes. Molecules 2006, 11, 792–807. [Google Scholar] [CrossRef]
- Marcos, I.S.; García, N.; Sexmero, M.J.; Hernández, F.A.; Escola, M.A.; Basabe, P.; Díez, D.; Urones, J.G. Synthetic studies towards picrasane quassinoids. Tetrahedron 2007, 63, 2335–2350. [Google Scholar] [CrossRef]
- Marcos, I.S.; Martínez, B.; Sexmero, M.J.; Diez, D.; Basabe, P.; Urones, J.G. Chemistry of ent-Halimic acid: synthesis of [4.3.3] propellanes. Synthesis 2006, 3865–3873. [Google Scholar] [CrossRef]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Díez, D.; Urones, J.G. Synthesis of quinone/hydroquinone sesquiterpenes. Tetrahedron 2010, 66, 8280–8290. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J.G. Synthesis of 12-epi-ent-polyalthenol an antitumor indole sesquiterpene alkaloid. Tetrahedron 2012, 68, 7932–7940. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Mollinedo, F.; Urones, J.G. Biomimetic synthesis of an antitumor indole sesquiterpene alkaloid, 12-epi-ent-pentacyclindole. Tetrahedron 2013, 69, 7285–7289. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Basabe, P.; Díez, D.; Gil, A.; Mollinedo, F.; Pérez-de la Rosa, F.; Pérez-Roth, E.; Padrón, J.M. Synthesis and biological activity of polyalthenol and pentacyclindole analogues. Eur. J. Med. Chem. 2014, 73, 265–279. [Google Scholar] [CrossRef]
- Marcos, I.S.; Moro, R.F.; Costales, I.; Escola, M.A.; Basabe, P.; Díez, D.; Urones, J.G. Synthesis of hexahydrocarbazoles by cyclisation of 3-(but-3-enyl) indole derivatives. Tetrahedron 2009, 65, 10235. [Google Scholar] [CrossRef]
- Bestmann, H.J. Phosphacumulene ylides and phosphaallene ylides [New synthetic methods (19)]. Angew. Chem. Int. Ed. 1977, 16, 349–364. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Lachance, N. A Mild, Efficient and general method for the synthesis of Trialkylsilyl (Z)-4-Oxo-2-alkenoates and γ-hydroxybutenolides. Synlett 1998, 1998, 31–32. [Google Scholar] [CrossRef]
- Rice, L.E.; Boston, M.C.; Finklea, H.O.; Suder, B.J.; Frazier, J.O.; Hudlicky, T. Regioselectivity in the reformatskii reaction of 4-bromocrotonate. role of the catalyst and the solvent in the normal vs. abnormal modes of addition to carbonyl substrates. J. Nat. Prod. 1984, 49, 1845–1848. [Google Scholar]
- LeGoff, E. Cyclopropanes from an easily prepared, highly active Zinc—Copper couple, dibromomethane, and olefins. J. Nat. Prod. 1964, 29, 2048–2050. [Google Scholar] [CrossRef]
- Fontana, A.; Ciavatta, M.L.; Cimino, G. Cladocoran A and B: Two novel γ-hydroxybutenolide sesterterpenes from the mediterranean coral Cladocora cespitosa. J. Org. Chem. 1998, 63, 2845–2852. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; McCarthy, P.J.; Kelly-Borges, M.; Lobkovsky, E.; Clardy, J. Dysidiolide: A novel protein phosphatase inhibitor from the caribbean sponge Dysidea etheria de laubenfels. J. Am. Chem. Soc. 1996, 118, 8759–8760. [Google Scholar] [CrossRef]
- Corey, E.J.; Roberts, B.E. Total Synthesis of Dysidiolide. J. Am. Chem. Soc. 1997, 119, 12425–12431. [Google Scholar] [CrossRef]
- Takahashi, M.; Dodo, K.; Sugimoto, Y.; Aoyagi, Y.; Yamada, Y.; Hashimoto, Y.; Shirai, R. Synthesis of the novel analogues of dysidiolide and their structure–activity relationship. Bioorg. Med. Chem. Lett. 2000, 10, 2571–2574. [Google Scholar] [CrossRef]
- Draetta, G.; Eckstein, J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta 1997, 1332, M53–M63. [Google Scholar] [CrossRef]
- Magnuson, S.R.; Sepp-Lorenzino, L.; Rosen, N.; Danishefsky, S.J. A concise total synthesis of dysidiolide through application of a dioxolenium-mediated diels−Alder Reaction. J. Am. Chem. Soc. 1998, 120, 1615–1616. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Cheng, Y.-X.; Robichaud, J. Total synthesis of (+)-dysidiolide. J. Org. Chem. 1998, 63, 228–229. [Google Scholar] [CrossRef]
- Brohm, D.; Metzger, S.; Bhargava, A.; Müller, O.; Lieb, F.; Waldmann, H. Natural Products Are Biologically Validated Starting Points in Structural Space for Compound Library Development: solid-phase synthesis of dysidiolide-derived phosphatase inhibitors. Angew. Chem. Int. Ed. 2002, 41, 307–311. [Google Scholar] [CrossRef]
- Kernan, M.R.; Faulkner, D.J. Regioselective oxidation of 3-alkylfurans to 3-alkyl-4-hydroxybutenolides. J. Nat. Prod. 1988, 53, 2773–2776. [Google Scholar] [CrossRef]
- Bradley, M.O.; Webb, N.L.; Anthony, F.H.; Devanesan, P.; Witman, P.A.; Hemamalini, S.; Chander, M.C.; Baker, S.D.; He, L.; Horwitz, S.B.; Swindell, C.S. Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin. Can. Res. 2001, 7, 3229–3238. [Google Scholar]
- Kuznetsova, L.; Chen, J.; Sun, L.; Wu, X.; Pepe, A.; Veith, J.M.; Pera, P.; Bernacki, R.J.; Ojima, I. Syntheses and evaluation of novel fatty acid-second-generation taxoid conjugates as promising anticancer agents. Bioorg. Med. Chem. Lett. 2006, 16, 974–977. [Google Scholar] [CrossRef]
- Pedersen, P.J.; Christensen, M.S.; Ruysschaert, T.; Linderoth, L.; Andresen, T.L.; Melander, F.; Mouritsen, O.G.; Madsen, R.; Clausen, M.H. Synthesis and biophysical characterization of chlorambucil anticancer ether lipid prodrugs. J. Med. Chem. 2009, 52, 3408–3415. [Google Scholar] [CrossRef]
- Faustino, M.; Consuelo, G.; Sonsoles, M.-S.; Federico, G. ET-18-OCH3 (Edelfosine): A selective antitumor lipid targeting apoptosis through intracellular activation of Fas/CD95 death receptor. Curr. Med. Chem. 2004, 11, 3163–3184. [Google Scholar]
- VanderRoest, J.M.; Grieco, P.A. Total synthesis of (±)-bruceantin. J. Nat. Prod. 1993, 115, 5841–5842. [Google Scholar] [CrossRef]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Quinone/Hydroquinone sesquiterpenes. Mini-Rev. Org. Chem. 2010, 7, 230–254. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Bridon, D.; Zardb, S.Z. The invention of radical chain reactions: Part XIV. A decarboxylative radical addition to quinones. Tetrahedron 1987, 43, 5307–5314. [Google Scholar] [CrossRef]
- Ling, T.; Poupon, E.; Rueden, E.J.; Theodorakis, E.A. Synthesis of (−)-Ilimaquinone via a radical decarboxylation and quinone addition reaction. Org. Lett. 2002, 4, 819–822. [Google Scholar] [CrossRef]
- Rosemeyer, H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na, K-atpase from the okinawa sea sponge Agelas sp.1). Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Hattori, T.; Adachi, K.; Shizuri, Y. New agelasine compound from the marine sponge Agelas mauritiana as an antifouling substance against macroalgae. J. Nat. Prod. 1997, 60, 411–413. [Google Scholar] [CrossRef]

























| Natural Source | Activity | Ref. | |
|---|---|---|---|
| Halim-1(10)-enes | |||
| Chettaphanin I, 1 | Adenochlaena siamensis (Syn. of Cladogynos orientalis) Croton crassifolius | [5,9,10,11,12,13] | |
| Ent-halimic acid methyl ester, 2 | Halimium viscosum (Villarino de los Aires, V.A.) | [14,15] | |
| 3 | H. viscosum (V.A.) | [14,15] | |
| 4 | H. viscosum (V.A.) | [14] | |
| 5 | H. viscosum (V.A.) | [14] | |
| 6 | H. viscosum (V.A.) | [14] | |
| 7 | H. viscosum (V.A.) | [14] | |
| 8 | H. viscosum (V.A.) Hymenaea courbaril | [15,16] | |
| 9 | H. viscosum (V.A.) | [14,15] | |
| 10 | H. viscosum (V.A.) | [15] | |
| 11 | H. viscosum (V.A.) | [15] | |
| 12 | H. viscosum (V.A.) | [17] | |
| 13 | H. viscosum (V.A.) | [18] | |
| 14 | H. viscosum (V.A.) | [18] | |
| 15 | H. viscosum (V.A.) | [14] | |
| 16 | H. viscosum (V.A.) | [14] | |
| 17 | H. viscosum (V.A.) | [18,19] | |
| 18 | H. viscosum (V.A.) | [18,19] | |
| 19 | H. viscosum (V.A.) | [18] | |
| 20 | H. viscosum (V.A.) | [18] | |
| 21 | H. viscosum (V.A.) | [18] | |
| 13R-Hydroxy-ent-halima-1(10),14-dien-18-oic acid, 22 | Hymenaea courbaril | A2780 human ovarian cell line (IC50 > 40 μg/mL) | [20] |
| 2S,13R-Dihydroxy-ent-halima-1(10),14-dien-18-oic acid, 23 | H. courbaril | [20] | |
| 2-Oxo-13R-hydroxy-ent-halima-1(10),14-dien-18-oic acid, 24 | H. courbaril | [20] | |
| 25 | H. viscosum (Celorico da Beira, C.B.) | [21] | |
| Crassifoliusin A, 26 | Croton crassifolius | Not cytotoxic (HepG2, SGC-7901 and K562) | [12] |
| Crassin D, 27 | C. crassifolius | Not cytotoxic (HL-60, and A549) | [22] |
| 28 | Cladogynos orientalis | Not cytotoxic/NCI-H187), not antitubercular (Mycobacterium tuberculosis H37Ra) | [13] |
| 29 | Hymenaea courbaril | [16] | |
| 30 | Halimium viscosum (La Fregeneda, L.F.) | [23] | |
| 31 | H. viscosum (V.A. and L.F.) | [14,23] | |
| 13R-Methyl-15-methoxy-ent-halim-1(10)-en-18-oate, 32 | H. viscosum (L.F. and C.B.) | [23,24] | |
| 33 | H. viscosum (L.F. and C.B.) | [24,25] | |
| 34 | H. viscosum (L.F. and C.B.) | [24] | |
| 35 | Hymenaea courbaril | [16] | |
| 36 | H. viscosum (C.B.) | [24] | |
| 37 | H. viscosum (C.B.) | [24] | |
| Crassifolin C, 38 | Croton crassifolius | HSV-1 (IC50 50 μg/mL) | [11] |
| 39 | H. viscosum (L.F. and C.B.) | [23,26] | |
| 18-Hydroxy-ent-halima-1(10),13E-dien-15-oic acid, 40 | Hymenaea stigonocarpa | [27] | |
| 41 | Eupatorium turbinatum (Syn. of Chromolaena bigelovii) | [28] | |
| 42 | Halimium viscosum | [15,29] | |
| 43 | H. viscosum | [17] | |
| 44 | H. viscosum | [15,29] | |
| 45 | H. viscosum | [29] | |
| 46 | H. viscosum | [29] | |
| 47 | Vellozia stipitata | [30] |
| Natural Source | Activity | Ref. | |
|---|---|---|---|
| Ent-halim-5-enes | |||
| 11R-Acetoxy-ent-halima-5,13E-dien-15-oic acid, 48 | Plectranthus ornatus | Enterococcus faecalis ATCC 51,299 MIC 15.63 Staphylococcus aureus CIP 106,760 MIC 15.63 | [31,32] |
| 49 | Haplopappus paucidentatus (Syn. of H. glutinosus) | [33] | |
| 50 | Relhania corymbosa R. squarrosa (Syn. of Oedera squarrosa) | [34] | |
| 51 | R. corymbosa R. squarrosa | [34] | |
| Salicifolic acid, 52 | Baccharis salicifolia (Syn. of B. salicina) | Germination inhibition of Panicum miliaceum (ECg50 1.9/mM) and Raphanus sativum (ECg50 4.6/mM) | [35] |
| Crothalimene B, 53 | Croton dichogamus | [36] | |
| 3α-Hydroxy-5,6-didehydrochiliolide, 54 | Chiliotrichum rosmarinifolium Nardophyllum bryoides | Murine lung adenocarcinoma cells (MLAC) LM3 (ID50 40 μM) Human ductal pancreatic carcinoma (HDPC) PANC1 (ID50 0.3μM) | [37,38] |
| Teupolin VIII, 55 | Teucrium polium | Human hepatoblastoma cells HepG2 (cell viability inhibition, 6.86% 100mM) | [39,40] |
| Teupolin VII, 56 | T. polium | HPC HepG2. CVI, 6.35% 100mM) | [39] |
| Halim-5-enes | |||
| Koanophyllic acid B, 57 | Koanophyllon conglobatum | [41] | |
| 58 | Haplopappus pulchellus | [42] | |
| 59 | H. pulchellus | [42] | |
| 60 | H. pulchellus | [42] | |
| 61 | H. pulchellus | [42] | |
| 62 | H. pulchellus | [42] | |
| 63 | H. pulchellus | [42] | |
| Koanophyllic acid A, 64 | Koanophyllon conglobatum | [41] | |
| 65 | Acalypha macrostachya | [43] | |
| 66 | A. macrostachya | [43] | |
| 67 | Colophospermum mopane | Klebsiella pneumoniae ATCC 13,883 (MIC 62.5 μg/mL) Escherichia coli ATCC 8739 (MIC 125 μg/mL) Enterococcus faecalis ATCC 29,212 (MIC 62.5 μg/mL) Staphylococcus aureus ATCC 25,923 (MIC 93.7 μg/mL) | [44] |
| Koanophyllic acid D, 68 | Koanophyllon conglobatum | [41] | |
| Koanophyllic acid C, 69 | K. conglobatum | [41] | |
| Micromonohalimane A, 70 | Micromonospora sp. | Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33,591 (MIC > 200 μg/mL) | [45] |
| Micromonohalimane B, 71 | Micromonospora sp. | (MRSA) ATCC 33,591 (MIC 40 μg/mL) | [45] |
| 8-Epi-halim-5-enes | |||
| Viteagnusin A 72 | Vitex agnus-castus | [46] | |
| Viterofolin D, 73 | V. rotundifolia | [47] | |
| Viterofolin E, 74 | V. rotundifolia | [47] | |
| 75 | Vellozia stipitata | [30] | |
| 76 | V. flavicans (Syn. of V. squamata) | [48] | |
| 77 | V. flavicans | [48] |
| Natural Source | Activity | Ref. | |
|---|---|---|---|
| Secohalimanes | |||
| Tessmannic acid, 78 | Tessmannia densiflora | Staphylococcus aureus, 11-20 mm Larvicidal activity Anophelles gambiae (LC50 48 h, 34 ppm) | [49,50] |
| Tessmannic acid methyl ester, 79 | T. densiflora | Larvicidal activity Anophelles gambiae (LC50 48 h, 92 ppm) | [49,50] |
| 80 | T. densiflora | [49,50] | |
| 81 | T. densiflora | [49,50] | |
| Secochiliotrin methyl ester, 82 | Chiliotrichum rosmarinifolium Nardophyllum lanatum | [37] | |
| Secochiliolide acid, 83 | C. rosmarinifolium N. lanatum N. bryoides | (MLAC) LM3 (ID50 28 μM) (HDPC) PANC1 (ID50 20 μM) | [37,38] |
| Secochiliolide acid methyl ester, 84 | C. rosmarinifolium N. bryoides | (MLAC) LM3 (ID50 14.1 μM) (HDPC) PANC1 (ID50 31.6 μM) | [37,38] |
| 19-Hydroxysecochiliolide acid, 85 | N. lanatum | [37] | |
| 19-Hydroxysecochiliolide acid methyl ester, 86 | N. lanatum | [37] | |
| Secochiliolide aldehyde, 87 | Chiliotrichum rosmarinifolium | [37] | |
| 88 | Nardophyllum bryoides | (MLAC) LM3 (ID50 20.0 μM) (HDPC) PANC1 (ID50 25.1 μM) | [38] |
| Teucvin (Mallotucin A), 89 | Teucrium viscidum Mallotus repandus Teucrium chamaedrys | [51,52,53,54,55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobal, I.E.; Roncero, A.M.; Moro, R.F.; Díez, D.; Marcos, I.S. The Methylene-Cycloalkylacetate (MCA) Scaffold in Terpenyl Compounds with Potential Pharmacological Activities. Molecules 2019, 24, 2120. https://doi.org/10.3390/molecules24112120
Tobal IE, Roncero AM, Moro RF, Díez D, Marcos IS. The Methylene-Cycloalkylacetate (MCA) Scaffold in Terpenyl Compounds with Potential Pharmacological Activities. Molecules. 2019; 24(11):2120. https://doi.org/10.3390/molecules24112120
Chicago/Turabian StyleTobal, Ignacio E., Alejandro M. Roncero, Rosalina F. Moro, David Díez, and Isidro S. Marcos. 2019. "The Methylene-Cycloalkylacetate (MCA) Scaffold in Terpenyl Compounds with Potential Pharmacological Activities" Molecules 24, no. 11: 2120. https://doi.org/10.3390/molecules24112120