Isolation, Chemical Profile and Antimalarial Activities of Bioactive Compounds from Rauvolfia caffra Sond
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profile
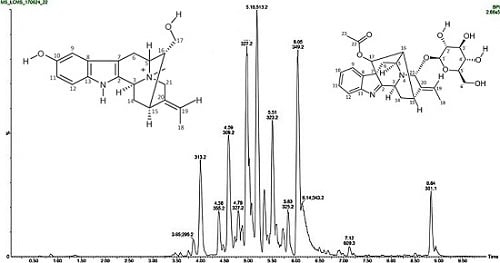
2.2. Isolation and Purification of Compounds
2.3. Antiplasmodial Activity
3. Materials and Methods
3.1. General Experimental Procedure
3.1.1. High-Resolution Mass Spectrometry
3.1.2. Infrared Spectroscopy
3.1.3. NMR Spectroscopy
3.1.4. Liquid Chromatography-Mass Spectrometry (LCMS) Analysis
3.2. Plant Collection and Preparation
3.3. Extraction of Plant Materials
3.4. Isolation and Purification of Compounds
3.5. Antiplasmodial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mboowa, G. Genetics of Sub-Saharan African Human Population: Implications for HIV/AIDS, Tuberculosis and Malaria. Int. J. Evol. Biol. 2014, 2014, 1–2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. The World Malaria Report. World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Mojarrab, M.; Shiravand, A.; Delazar, A.; Afshar, H.F. Evaluation of in vitro antimalarial activity of different extracts of Artemisia aucheri Boiss and A. armeniaca Lam and fractions of the most potent extracts. Sci. World J. 2014, 2014, 1–6. [Google Scholar]
- Chukuocha, U.M.; Nwakwuo, G.C.; Alinnor, L.C. Influence of maternal factors on neonatal malaria in South Eastern Nigeria. J. Dis. Global Health 2016, 7, 71–77. [Google Scholar]
- Jigam, A.A.; Abdulrazaq, U.T.; Egbuta, M.N. In-vivo antimalarial and toxicological evaluation of Chrozophora senegalensis A. uss (Euphorbiaceae) extracts. J. Appl. Pharmacol. Sci. 2011, 1, 90–94. [Google Scholar]
- World Health Organisation. The World Malaria Report. World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organisation. Guidelines for the Treatment of Malaria, 3rd Ed. ed; World Health Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- Schwikkard, S.; van Heerden, F.R. Antimalarial activity of plant metabolites. Nat. Prod. Rep. 2002, 19, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Pant, N.; Jain, D.C.; Bhakuni, R.S. Antimalarial agents from plant sources. Current Science 2003, 85, 1314–1329. [Google Scholar]
- Frederich, M.; Tits, M.; Angenot, L. Potential antimalarial activity of indole alkaloids. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Jain, M.; Kaur, T.; Jain, R. Antimalarials from nature. Bioorg. Med. Chem. 2009, 17, 3229–3256. [Google Scholar] [CrossRef]
- Batista, R.; de Jesus Silva Júnior, A.; de Oliveira, A.B. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part II. Non alkaloidal natural products. Molecules 2009, 14, 3037–3072. [Google Scholar]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [Green Version]
- Fennell, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rukunga, G.; Gathirwa, J.; Omar, S.; Muregi, F.; Muthaura, C.; Kirira, P.; Mungai, G.; Kofi-Tsekpo, W. Anti-plasmodial activity of the extracts of some Kenyan medicinal plants. J. Ethnopharmacol. 2009, 121, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkunya, M.H. Progress in the search for Antimalarials. In NAPRECA Monograph Series No.4.; NAPRECA: Addis Ababa, Ethiopia, 1992; Addis Ababa University. [Google Scholar]
- Tshikalange, T.E.; Meyer, J.J.M.; Hussein, A.A. Antimicrobial activity, toxicity and the Isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J. Ethnopharmacol. 2005, 96, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, G.H.; Gurib-Fakim, A. Plant Resources of Tropical Africa 11(1): Medicinal Plants 1; PROTA Foundation/Backhuys: Leiden, The Netherlands, 2008; pp. 480–483. [Google Scholar]
- Njau, E.A. An ethnobotanical study of medicinal plants used by the Maasai People of Manyara, Arusha-Tanzania. Master’s Thesis, School of Graduate Studies. Addis Ababa University, Addis Ababa, Ethiopia, 2001. [Google Scholar] [CrossRef]
- Bryant, A.T. Zulu Medicine and Medicine-Men, 2nd Ed. ed; Struik: Cape Town, South Africa, 1996. [Google Scholar]
- Amole, O.O.; Onabanjo, A.C.; Agbaje, E.C. Effect of bark extract of Rauvolfia vomitoria (Afzel) in malaria. Parasitol. Internat. 1998, 47, 283–289. [Google Scholar]
- Campbell, J.I.A.; Mortensen, A.; Mølgaard, P. Tissue lipid lowering effect of traditional Nigerian antidiabetic infusion of Rauvolfia vomitoria foliage and Citrus aurantium fruit. J. Ethnopharmacol. 2006, 104, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pesewu, G.A.; Cutler, R.R.; Humber, D.P. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. J. Ethnopharmacol. 2008, 116, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, L. Drugless Healing Secrets; Panse Press: Ibadan, Nigeria, 2007. [Google Scholar]
- McMillen, H. The adapting healer: Pioneering through shifting epidemiological and social cultural landscapes. Soc. Sci. Med. 2004, 59, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Siddiqui, S. The subsidiary alkaloids of Rauwolfia vomitoria Afzuelia. Pak. J. Sci. Ind. Res. 1979, 22, 121–123. [Google Scholar]
- Amer, M.M.; Court, W.E. Leaf alkaloids of Rauwolfia vomitoria. Phytochemistry 1980, 19, 1833–1836. [Google Scholar] [CrossRef]
- Katič, M.; Kušan, E.; Prošek, M.; Bano, M. Quantitative densitometric determination of reserpine and ajmaline in Rauwolfia vomitoria by HPTLC. J. High Res. Chromatogr. 1980, 3, 149–150. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal natural products: A biosynthetic approach, 2nd Ed. ed; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics and color of Cabernet Franc, Merlot, and Pinot Noir wine from British Columbia. J. Agricul. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Brit. J. Nutri. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.F.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Khan, M.A.; Siddiqui, S. Studies in the Alkaloids of Rauwolfia caffra Sonder. Part I. Isolation of Ajmalicine, Ajmaline, Raucaffrine and Three New Alkaloids: Raucaffricine, Raucaffriline and Raucaffridine. Pak. J. Sci. Ind. Res. 1965, 8, 23. [Google Scholar]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlhapi, D.B.; Ramaite, I.D.I.; Van Ree, T.; Anokwuru, C.P.; Orazio, T.-S.; Hoppe, H.C. Isolation, Chemical Profile and Antimalarial Activities of Bioactive Compounds from Rauvolfia caffra Sond. Molecules 2019, 24, 39. https://doi.org/10.3390/molecules24010039
Tlhapi DB, Ramaite IDI, Van Ree T, Anokwuru CP, Orazio T-S, Hoppe HC. Isolation, Chemical Profile and Antimalarial Activities of Bioactive Compounds from Rauvolfia caffra Sond. Molecules. 2019; 24(1):39. https://doi.org/10.3390/molecules24010039
Chicago/Turabian StyleTlhapi, Dorcas B., Isaiah D. I. Ramaite, Teunis Van Ree, Chinedu P. Anokwuru, Taglialatela-Scafati Orazio, and Heinrich C. Hoppe. 2019. "Isolation, Chemical Profile and Antimalarial Activities of Bioactive Compounds from Rauvolfia caffra Sond" Molecules 24, no. 1: 39. https://doi.org/10.3390/molecules24010039