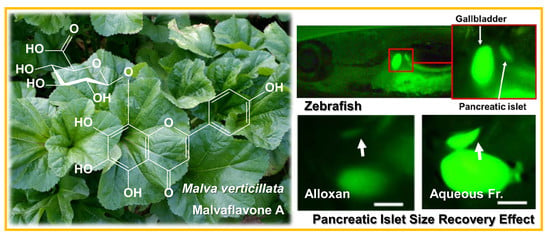
Flavonoid 8-O-Glucuronides from the Aerial Parts of Malva verticillata and Their Recovery Effects on Alloxan-Induced Pancreatic Islets in Zebrafish
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Reagents and Instrumentation
3.3. Isolation of Flavonoids
3.4. Zebrafish Care and Maintenance
3.5. Evaluation of the Toxicity in Zebrafish Embryo
3.6. Evaluation of Recovery Efficacy for AX-Induced PI Damage in Zebrafish Larvae
3.7. Action of DZ on AX-induced Diabetic Zebrafish
3.8. Statistical Analysis
3.9. DPPH RS Activity
3.10. ABTS RS Activity
3.11. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.12. Superoxide Scavenging Activity
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arkhtar, K.; Shah, S.W.A.; Shak, A.A.; Haleem, S.K.; Sultana, N. Pharmacological effect of Rubus ulmifolius Schott as antihyperglycemic and antihyperlipidemic on streptozotocin (STZ)-induced albino mice. Appl. Biol. Chem. 2017, 60, 411–418. [Google Scholar] [CrossRef]
- Rhodes, C.J. Type 2 diabetes-a matter of β-cell life and death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renstroöm, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J.; White, M.F. Molecular insights into insulin action and secretion. Eur. J. Clin. Invest. 2002, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Mollet, B.; Rowland, I. Functional foods: At the frontier between food and pharma. Curr. Opin. Iotechnol. 2002, 13, 483–485. [Google Scholar] [CrossRef]
- Roberfroid, M.B. A European consensus of scientific concepts of functional foods. Nutrition 2000, 16, 689–691. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Role of bioactive food components in diabetes prevention: Effects on beta-cell function and preservation. Nutr. Metab. Insights 2014, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects and Bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proceed. Natl. Acad. 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Odontuya, G.; Enkhmaa, G.; Batbayar, N.; Naran, R.; Inngjerdingen, K.T.; Michaelsen, T.E.; Paulsen, B.S. Pharmacological activities of a mongolian medicinal plant, Malva mohileviensis Down. Eur. J. Med. Plant 2012, 2, 230–241. [Google Scholar] [CrossRef]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Kanari, M. Characterization of an acidic polysaccharide from the seeds of Malva verticillata stimulating the phagocytic activity of cells of the RES1. Planta Med. 1990, 56, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Cho, S.M.; Joo, S.W.; Kim, H.G.; Lee, Y.G.; Kang, S.C.; Baek, N.I. Glycosyl glycerides from the aerial parts of Malva verticillata and their chemopreventive effects. Bioor. Chem. 2018. In press. [Google Scholar] [CrossRef]
- Ko, J.H.; Castaneda, R.; Joo, S.W.; Kim, H.G.; Lee, Y.G.; Lee, Y.H.; Kang, T.H.; Baek, N.I. Glycerides isolated from the aerial parts of Malva verticillata cause immunomodulation effects via splenocyte function and NK anti-tumor activity. Food Sci. Biotechnol. 2018, 1–8. [Google Scholar] [CrossRef]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.; Singh, A.K. Hypoglycaemic effect of galactooligosaccharides in alloxan-induced diabetic rats. J. Dairy Res. 2015, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kawai, K.; Itakura, M. Effects of fructo-oligosaccharides on blood glucose and serum lipids in diabetic subjects. Nutr. Res. 1984, 4, 961–966. [Google Scholar] [CrossRef]
- Casirola, D.M.; Ferraris, R.P. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metab. Clin. Exp. 2006, 55, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Agheli, N.; Kabir, M.; Berni-Canani, S.; Petitjean, E.; Boussairi, A.; Luo, J.; Bornet, F.; Salma, G.; Rizkalla, S.W. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. J. Nutr. 1998, 128, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dong, T.; Zhang, A.; Yang, J.; Yan, G.; Sakurai, T.; Wu, X.; Han, Y.; Wang, X. Pharmacokinetics of hesperetin and naringenin in the Zhi ZhuWan, a traditional Chinese medicinal formulae, and its pharmacodynamics study. Phytother. Res. 2013, 27, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.L.S.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Kataja-Tuomola, M.K.; Kontto, J.P.; Männistö, S.; Albanes, D.; Virtamo, J. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur. J. Clin. Nutr. 2011, 65, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M. Tea flavonoids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17, 288–290. [Google Scholar] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Lee, J.E.; Kyung, M.J.; Youn, J.H.; Oh, J.B.; Whang, W.K. Anti-diabetic functional food with wasted litchi seed and standard of quality control. Appl. Biol. Chem. 2017, 60, 197–204. [Google Scholar] [CrossRef]
- Billeter, M.; Meier, B.; Sticher, O. 8-Hydroxyflavonoid glucuronides from Malva sylvestris. Phytochemistry 1991, 30, 987–990. [Google Scholar] [CrossRef]
- Wolbiś, M. Flavonol glycosides from Sedum album. Phytochemistry 1989, 28, 2187–2189. [Google Scholar] [CrossRef]
- Sikorska, M.; Matławska, I. 8-Hydroxyflavonoid glucuronides of Malope trifida. Acta Physiol. Plant. 2004, 26, 291–297. [Google Scholar] [CrossRef]
- Iida, N.; Inatomi, Y.; Murata, H.; Inada, A.; Murata, J.; Lang, F.A.; Matsuura, N.; Nakanishi, T. A new flavone xyloside and two new flavan-3-ol glucosides from Juniperus communis var. depressa. Chem. Biodivers. 2007, 4, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Ji, M.G.; Kim, K.; Kim, U.J.; Kang, T.H. Synergistic potentials of coffee on injured pancreatic islets and insulin action via KATP channel blocking in zebrafish. J. Agric. Food Chem. 2015, 63, 5612–5621. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Le, H.T.; Rodriguez, I.; Kim, E.Y.; Kim, K.; Jeong, S.Y.; Woo, S.H.; Lee, Y.R.; Castañeda, R.; Hong, J.; et al. Enhanced antidiabetic efficacy and safety of compound K⁄ β-cyclodextrin inclusion complex in zebrafish. J. Ginseng Res. 2017, 41, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Phadatare, P.D.; Chandrashekhar, V.M. Influence of esomeprazole on hypoglycemic activity of oral antidiabetic agents in rats and rabbits. Mol. Cell. Biochem. 2011, 354, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Saito, M.; Oh, K.B.; Nemoto, Y.; Matsuoka, H.; Natsume, M.; Abe, H. Intracellular fate of 2-NBDG, a fluorescent probe for glucose uptake activity, in Escherichia coli cells. Biosci. Biotechnol. Biochem. 1996, 60, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, D.W.; Kim, W.H.; Um, J.I.; Yim, S.H.; Oh, W.K.; Williams, D.R. Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem. Boil. 2013, 8, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Liu, C.; Chan, C.B.; Ye, K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 2016, 5, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test Guideline 236: Guideline for Testing of Chemicals, Fish Embryo Acute toxicity (Fet) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Hu, X. Enhancement of polyphenol content and antioxidant capacity of oat (Avena nuda L.) bran by cellulase treatment. Appl. Biol. Chem. 2016, 59, 397–403. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agr. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound 1 is available from the authors. |




| Compounds | DPPH EC50 a (μM) | ABTS EC50 (μM) | ORAC (µmol TE/ µmol) | SOD EC50 (μM) |
|---|---|---|---|---|
| Quercetin | 4.08 ± 0.36 b | 1.73 ± 0.09 | 7.33 ± 0.15 | 0.43 ± 0.10 |
| BHA | 8.33 ± 0.46 | 4.65 ± 0.18 | 2.86 ± 0.26 | 0.44 ± 0.09 |
| 1 | >50 | 2.22 ± 0.05 | 14.38 ± 0.35 | 0.73 ± 0.09 |
| 2 | >50 | 3.38 ± 0.15 | 8.06 ± 0.36 | 1.51 ± 0.15 |
| 3 | 5.98 ± 0.24 | 1.52 ± 0.04 | 12.48 ± 1.27 | 0.98 ± 0.13 |
| 4 | 31.79 ± 2.22 | 4.51 ± 0.13 | 6.56 ± 0.32 | 1.04 ± 0.21 |
| 5 | 33.80 ± 1.89 | 4.05 ± 0.14 | 6.42 ± 0.18 | 0.70 ± 0.18 |
| 6 | >50 | 21.62 ± 1.26 | 3.83 ± 0.30 | 1.31 ± 0.20 |
| Carbon No. | 1 | |
|---|---|---|
| δc | δH | |
| Aglycon | ||
| 2 | 163.0 | – |
| 3 | 100.5 | 6.57 (s) |
| 4 | 180.7 | – |
| 5 | 148.3 | – |
| 6 | 131.2 | – |
| 7 | 144.1 | – |
| 8 | 127.9 | – |
| 9 | 156.0 | – |
| 10 | 100.8 | – |
| 1′ | 121.0 | – |
| 2′, 6′ | 128.4 | 8.25 (d, 7.6) |
| 3′, 5′ | 115.7 | 7.35 (d, 7.6) |
| 4′ | 161.6 | – |
| Sugar moiety | ||
| 1′′ | 101.1 | 5.05 (d, 7.2) |
| 2′′ | 74.7 | 4.02–4.04 b |
| 3′′ | 78.6 | 4.02–4.04 b |
| 4′′ | 71.3 | 4.20 (m) |
| 5′′ | 75.7 | 3.82 (d, 8.8) |
| 6′′ | 174.0 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-H.; Nam, Y.H.; Joo, S.-W.; Kim, H.-G.; Lee, Y.-G.; Kang, T.H.; Baek, N.-I. Flavonoid 8-O-Glucuronides from the Aerial Parts of Malva verticillata and Their Recovery Effects on Alloxan-Induced Pancreatic Islets in Zebrafish. Molecules 2018, 23, 833. https://doi.org/10.3390/molecules23040833
Ko J-H, Nam YH, Joo S-W, Kim H-G, Lee Y-G, Kang TH, Baek N-I. Flavonoid 8-O-Glucuronides from the Aerial Parts of Malva verticillata and Their Recovery Effects on Alloxan-Induced Pancreatic Islets in Zebrafish. Molecules. 2018; 23(4):833. https://doi.org/10.3390/molecules23040833
Chicago/Turabian StyleKo, Jung-Hwan, Youn Hee Nam, Sun-Woo Joo, Hyoung-Geun Kim, Yeong-Geun Lee, Tong Ho Kang, and Nam-In Baek. 2018. "Flavonoid 8-O-Glucuronides from the Aerial Parts of Malva verticillata and Their Recovery Effects on Alloxan-Induced Pancreatic Islets in Zebrafish" Molecules 23, no. 4: 833. https://doi.org/10.3390/molecules23040833