Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Contents
2.2. Antioxidant Capacity
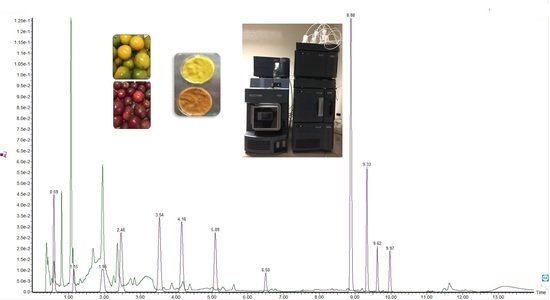
2.3. Identification of Bioactive Compounds by UPLC-MS
2.4. Correlation Between Bioactive Compounds and Antioxidant Capacity
3. Materials and Methods
3.1. Reagents and Standards
3.2. Sample Preparation and Extraction
3.3. Content of Total Phenolic Compounds
3.4. Content of Total Flavonoids
3.5. Trolox Equivalent Antioxidant Capacity (TEAC)
3.6. Radical Scavenging Capacity Using the DPPH Method
3.7. Evaluation of the Protective Effect on Human Erythrocytes
3.8. UPLC-MS Analysis of Bioactive Compounds
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Durán, R.; Valenzuela, A. La experiencia japonesa con los alimentos foshu: ¿Los verdaderos alimentos funcionales? Rev. Chil. Nutr. 2010, 37, 224–233. [Google Scholar] [CrossRef]
- Coronado, M.; Vega-León, S.; Gutiérrez, R.; Vázquez, M.; Radilla, C. Antioxidantes: perspectiva actual para la salud humana. Rev. Chil. Nutr. 2015, 42, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Murillo, E.; Britton, G.B.; Durant, A.A. Antioxidant activity and polyphenol content in cultivated and wild edible fruits grown in Panama. J. Pharm. Bioallied. Sci. 2012, 4, 313–317. [Google Scholar] [PubMed]
- Jia, Z.; Dumont, M.J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultrahigh-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Maldonado-Astudillo, Y.I.; Alia-Tejacal, I.; Núñez-Colín, C.A.; Jiménez-Hernández, J.; Pelayo-Zaldívar, C.; López-Martínez, V.; Andrade-Rodríguez, M.; Bautista-Baños, S.; Valle-Guadarrama, S. Postharvest physiology and technology of Spondias purpurea L. and S. mombin L. Sci. Hort. 2014, 174, 193–206. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [Green Version]
- Allwood, J.W.; Goodacre, R. An introduction to liquid chromatography–mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochem. Anal. 2010, 21, 33–47. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Zhang, A.; Wang, P.; Han, Y. Ultra-performance liquid chromatography coupled to mass spectrometry as a sensitive and powerful technology for metabolomic studies. J. Sep. Sci. 2011, 34, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Sánchez, C.; Pascual, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Ávila, S.; Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C.W.I. The association between chromaticity, phenolics, carotenoids, and in vitro antioxidant activity of frozen fruit pulp in Brazil: An application of chemometrics. J. Food Sci. 2014, 79, C510–C516. [Google Scholar] [CrossRef] [PubMed]
- Bazílio-Omena, C.M.; Barros-Valentim, I.; Da Silva-Guedes, G.; Rabelo, L.A.; Mano, C.M.; Henriques-Bechara, E.J.; Sawaya, A.; Salles-Trevisan, M.T.; Gomes-Da Costa, J.; Silva-Ferreira, R.C.; et al. Antioxidant, anti-acetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seeds of exotic Brazilian fruits: antioxidant, anti-acetylcholinesterase and cytotoxic activities in fruits. Food Res. Int. 2012, 49, 334–344. [Google Scholar] [CrossRef]
- Araújo-Da Silva, A.R.; Maia-De Morais, S.; Mendes-Marques, M.M.; Ferreira-De Oliveira, D.; Costa-Barros, C.; De Almeida, R.R.; Pinto-Vieira, I.G.; Florindo-Guedes, M.I. Chemical composition, antioxidant and antibacterial activities of two Spondias species from Northeastern Brazil. Pharm. Biol. 2012, 50, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; Vargas y Vargas, M.L.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Beserra-Almeida, M.M.; Machado-de-Sousa, P.H.; Campos-Arriaga, Â.M.; Matias-do-Prado, G.; de Carvalho-Magalhães, C.E.; Arraes-Maia, G.; Gomes-de Lemos, T.L. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton L., D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.I.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Noruma, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Chawla, R.; Narula, A.; Goel, R.; Arora, R.; Sharma, R.K. Anti-hemolytic, hemagglutination inhibition and bacterial membrane disruptive properties of selected herbal extracts attenuate virulence of Carbapenem Resistant Escherichia coli. Microb. Pathog. 2016, 95, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis). Food Chem. Toxicol. 2009, 47, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Silva, R.V.; Costa, S.C.; Branco, C.R.; Branco, A. In vitro photoprotective activity of the Spondias purpurea L. peel crude extract and its incorporation in a pharmaceutical formulation. Ind. Crops Prod. 2016, 83, 509–514. [Google Scholar] [CrossRef]
- Theodoridis, G.A.; Gika, H.G.; Want, E.J.; Wilson, I.D. Liquid chromatography–mass spectrometry based global metabolite profiling: A review. Anal. Chim. Acta 2012, 711, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Olveira-Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef]
- Cortés, C.; Esteve, M.J.; Frígola, A.; Torregrosa, F. Identification and quantification of carotenoids including geometrical isomers in fruit and vegetable juices by liquid chromatography with ultraviolet−diode array detection. J. Agric. Food Chem. 2004, 52, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. J. Sci. Food Agric. 2008, 88, 294–304. [Google Scholar] [CrossRef]
- Roux, A.; Lison, D.; Junot, C.; Heilier, J.F. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clin. Biochem. 2011, 44, 119–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida-Melo, E.; Sucupira-Maciel, M.I.; Galvão-de Lima, V.L.A.; do Nascimento, R.J. Capacidade antioxidante de frutas. Rev. Bras. Ciênc. Farm. 2008, 44, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macia, A.; Motilva, M.J. Methods for preparing phenolic extracts from olive cake for potential application as food antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Ornelas-Paz, J.J.; Gardea-Béjar, A.A.; Yahia, E.M.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Ruiz-Cruz, S.; Ibarra-Junquera, V. Phenolic compounds of hawthorm (Crataegus spp.): the biological activity associated to the protection of human health. Rev. Fit. Mex. 2018, 41, 339–349. [Google Scholar]
- Ornelas-Paz, J.J.; Martínez-Burrola, J.M.; Ruiz-Cruz, S.; Santana-Rodríguez, V.; Ibarra-Junquera, V.; Olivas, G.I.; Pérez-Martínez, J.D. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chem. 2010, 119, 1619–1625. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez- Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, X.; Yang, X.H.; Qiu, N.X.; Wang, Y.; Wang, Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Moein, S.; Moein, M.R. Relationship between antioxidant properties and phenolics in Zhumeria majdae. J. Med. Plants Res. 2010, 4, 517–521. [Google Scholar]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2013, 55, 117–123. [Google Scholar] [CrossRef]
Sample Availability: Samples of the phenolic compounds and plum extracts are available from the authors. |




| Compound | Red Plum (mg/100 g of Extract) | Yellow Plum (mg/100 g of Extract) | ||
|---|---|---|---|---|
| Methanol | Ethanol | Methanol | Ethanol | |
| Chlorogenic acid | 11.15 ± 0.73 b | 4.44 ± 0.34 b | 3.78 ± 0.07 d | 2.40 ± 0.09 b |
| Gallic acid | 6.38 ± 0.09 c | 3.11 ± 0.06 c | 6.82 ± 0.05 b | 4.06 ± 0.77 a |
| Resorcinol | 12.89 ± 1.06 a | 5.02 ± 0.63 a | 8.54 ± 0.68 a | 2.63 ± 0.62 ab |
| Rutin | 7.70 ± 0.19 c | 3.90 ± 0.17 b | 5.37 ± 0.50 c | 3.03 ± 0.01ab |
| Bioactive Compound | Red (Rt) | Yellow (Rt) | MM (g/mol) | m/z (M + H)+ | m/z (M + H)− | % of Area |
|---|---|---|---|---|---|---|
| Vanillic acid | 0.39 | N.D. | 168 | N.D. | 167 | 7.00 |
| Cyanidin | 0.51 | N.D. | 287 | 288 | 286 | 6.33 |
| Glycitein | 0.51 | 0.57 | 284 | 285 | N.D. | 3.06 |
| Catechin | 1.23 | 1.25 | 290 | 291 | 289 | 3.93 |
| Physcion | 2.54 | 2.56 | 284 | 285 | N.D. | 2.24 |
| Kaempferide | 2.54 | 2.56 | 300 | 301 | N.D. | 2.24 |
| 3-Cafeoylquinic acid | 3.50 | N.D. | 354 | 355 | 353 | 1.55 |
| Myricitrin | 4.19 | 4.23 | 464 | 465 | N.D. | 3.28 |
| β-Cryptoxanthin | 5.13 | N.D. | 552 | 553 | 551 | 1.98 |
| Ellagic acid | 5.55 | N.D. | 301 | 303 | N.D. | 1.59 |
| Luteolin | 6.30 | N.D. | 286 | 287 | N.D. | 2.63 |
| Epigallocatechin | 6.61 | 6.66 | 458 | 459 | N.D. | 1.48 |
| Epigallocatechin gallate | 6.61 | N.D. | 458 | 459 | N.D. | 0.97 |
| Hesperetin | 6.61 | 6.66 | 302 | 303 | 301 | 1.48 |
| Quercitrin | 6.77 | 6.84 | 448 | 449 | 447 | 2.10 |
| Isorhamnetin | 7.31 | N.D. | 302 | 303 | N.D. | 0.96 |
| Baicalein/Galangin | 7.31 | N.D. | 270 | 271 | N.D. | 2.33 |
| Chalcone | 8.26 | 8.3 | 208 | 209 | N.D. | 2.48 |
| Quercetin | 9.26 | 9.29 | 302 | 303 | 301 | 0.78 |
| Bioactive Compounds | Red Plum | Yellow Plum | ||||
|---|---|---|---|---|---|---|
| ABTS | DPPH | Hemolysis | ABTS | DPPH | Hemolysis | |
| Phenols | 0.8810 | 0.7800 | 0.6907 | 0.9997 | 0.8465 | 0.9686 |
| Flavonoids | 0.8130 | 0.6934 | 0.5812 | 0.9879 | 0.9096 | 0.9930 |
| Chlorogenic acid | 0.8581 | 0.7503 | 0.6461 | 0.9754 | 0.6922 | 0.8794 |
| Gallic acid | 0.9952 | 0.9608 | 0.9102 | 0.9520 | 0.6256 | 0.8338 |
| Resorcinol | 0.9139 | 0.8247 | 0.7337 | 0.8877 | 0.9945 | 0.9791 |
| Rutin | 0.8868 | 0.7878 | 0.6899 | 0.9520 | 0.6256 | 0.8339 |
| Bioactive Compounds | Red Plum | Yellow Plum | ||||
|---|---|---|---|---|---|---|
| ABTS | DPPH | Hemolysis | ABTS | DPPH | Hemolysis | |
| Phenols | 0.9378 | 0.9857 | 0.8651 | 0.7794 | 0.8102 | 0.7257 |
| Flavonoids | 0.9997 | 0.8773 | 0.9812 | 0.9918 | 0.9970 | 0.9781 |
| Chlorogenic acid | 0.9099 | 0.9954 | 0.8261 | 0.9983 | 0.9941 | 0.9997 |
| Gallic acid | 0.9336 | 0.6286 | 0.9808 | 0.9972 | 0.9921 | 0.9999 |
| Resorcinol | 0.9828 | 0.9435 | 0.9370 | 0.7692 | 0.8007 | 0.7146 |
| Rutin | 1.0000 | 0.8660 | 0.9854 | 0.9039 | 0.9245 | 0.8660 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. https://doi.org/10.3390/molecules23123200
Hernández-Ruiz KL, Ruiz-Cruz S, Cira-Chávez LA, Gassos-Ortega LE, Ornelas-Paz JdJ, Del-Toro-Sánchez CL, Márquez-Ríos E, López-Mata MA, Rodríguez-Félix F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Molecules. 2018; 23(12):3200. https://doi.org/10.3390/molecules23123200
Chicago/Turabian StyleHernández-Ruiz, Karen L., Saul Ruiz-Cruz, Luis A. Cira-Chávez, Laura E. Gassos-Ortega, José de Jesús Ornelas-Paz, Carmen L. Del-Toro-Sánchez, Enrique Márquez-Ríos, Marco A. López-Mata, and Francisco Rodríguez-Félix. 2018. "Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS" Molecules 23, no. 12: 3200. https://doi.org/10.3390/molecules23123200