2.1. Nutraceutical Properties
Food processing plays an important role in the bioactive compound degradation, because several transformations of phenolics occur to produce yellowish or brownish pigments [
18]: the final product outward appearance is a crucial in determination of consumers’ choices and anthocyanins are the main food colorants responsible for intense colour (associated with raw material freshness and good quality) [
19]. Moreover, physical and biological factors as temperature increase and enzymatic activity may be very important in degradation of polyphenolic compounds [
10].
In this research, the used methods allowed a rapid measurement of TAC and TPC: the Folin-Ciocalteu method suffers many interferences but it can be a complementary technique applied to confirm and support chromatography results, as reported in this study. The TPC ranged from 10.98 ± 2.18 mg
GAE/100g
Pr (mango jam, CM2) to 625.34 ± 67.86 mg
GAE/100g
Pr (red guava jam, CM8), while TAC ranged from 0.59 ± 0.25 mg
C3G/100g
Pr (mango jam, CM2) to 9.56 ± 0.46 mg
C3G/100g
Pr (orange jam, CM7): values obtained from the analysed extracts (
Table 1) were higher than values reported by Poiana et al. [
20] and Rababah et al. [
21]; the differences in phenolic and anthocyanin content could be due to the effects of several internal and external factors on plant material (genetic variability, climatic conditions and environmental factors) [
22]. In particular, Comoros pedoclimatic conditions such as volcanic soil, high temperatures, well distributed rains, influence polyphenolic content in fresh fruits and related products.
Bioactive compound redox properties allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers [
23]. In this research, the Ferric Reducing Antioxidant Power (FRAP) assay was used to evaluate antioxidant capacity of fruit jams and jellies, studying the ability of antioxidants to reduce Fe
3+ ions to Fe
2+ ions. Jams and jelly FRAP value ranged from 4.71 ± 2.07 mmol Fe
2+ kg
Pr−1 (mango jam, CM1) to 25.50 ± 0.28 mmol Fe
2+ kg
Pr−1 (red guava jam, CM8) as shown in
Table 1, in accordance with other studies [
24,
25].
Antioxidant activity of fruit-derived products was determined by different bioactive molecules (e.g., polyphenols, as anthocyanins and vitamin C) [
26]; for example, antioxidant activity of cyanidin is about 4 times higher compared to ascorbic acid [
27]. In this study, the TPC/TAC/antioxidant activity correlation was positive: results showed a significant Pearson correlation coefficients (R = 0.6636 for TPC/antioxidant activity and R = 0.4347 for TAC/antioxidant activity).
2.2. Phytochemical Composition
Antioxidant (polyphenols and vitamin C) and anti-inflammatory (terpenes) compounds are the main biologically active substances in fresh fruits and derived products: synergistic or additive health-promoting effects of different phytochemicals (phytocomplex) contribute to biological activity better than a single molecule or a group of few compounds [
28]. In this study 22 biologically active compounds together with 9 nutritional substances were selected as markers for HPLC fingerprinting due to their importance in humans [
29]. In
Supplementary material (Suppl. Figure S1) HPLC chromatograms of orange jams are reported as an example of analysed fruit-based products.
An important question in HPLC analysis is whether the peak comprises one or more components. In quality control and research analysis, impurities hidden behind the peak of interest can falsify results and an undetected component might lead to a loss of essential information. In this research, a peak purity check was assessed in order to control if peaks were pure or contained impurities comparing spectra recorded during the elution of each peak. No-coeluting peaks were detected. Moreover, HPLC-DAD does not allow a definitive identification of phytochemicals. Indeed, liquid chromatography (LC) coupled to mass (MS) or mass/mass spectrometry (MS2) is one of the most effective technique for analysis on complex plant extract/fresh fruit/derived product providing a rapid and accurate identification of phytochemicals, as phenolics. For this reason, future developments are necessary but in this preliminary study HPLC-DAD was a simply, rapid and effective approach to describe considered samples in relation to the research aim. Additional markers with demonstrated biological activity could be also taken into consideration for a better identification of the chromatographic pattern of fruit-derived products, together with a mass spectrometry detection of unknown peaks.
The chemical fingerprint of analysed jams and jellies is reported in
Table 2,
Table 3 and
Table 4 (phenolics, other health-promoting components and nutritional substances, respectively). The total bioactive compound content (TBCC) was calculated as the sum of the main molecules (polyphenols, monoterpenes and vitamin C) selected for their biological proved effects on humans and detected in the extracts: TBCC value ranged from 94.25 ± 4.13 mg/100 g
Pr (banana flower, CM4) to 357.50 ± 2.10 mg/100 g
Pr (orange jam, CM7). This is only a preliminary study on tropical fruit-based jams: in further fingerprint studies, other markers with health-promoting capacity or positive nutritional value should be added for a complete chromatographic pattern characterisation coupling a mass spectrometry (MS) detection of unknown peaks to a UV-visible determination.
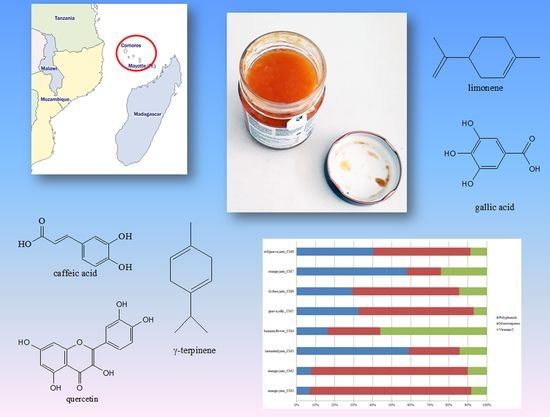
In
Figure 1 identified health-promoting substances were grouped into bioactive classes for the evaluation of the single contribution of each class to total phytocomplex/TBCC (mean values were considered). The most important class in tamarind (CM3) and orange (CM6) jams was polyphenols (58.93% and 58.07%, respectively), expressed as the sum of anthocyanins, phenolic acids, flavanols, catechins and tannins, while monoterpenes were the first class in mango jam (83.79%), guava jelly (60.48%) and lychee jam (56.04%). Banana flower showed a high percentage of vitamin C (56.05%), while red guava jam presented similar content of polyphenols and monoterpenes (40.37% and 50.93%, respectively) with a positive percentage of vitamin C (8.70%).
Results showed that analysed fruit jams and jellies are a very good source of polyphenolic compounds.
Figure 2 reports the single contribution of each polyphenolic class to total polyphenols detected by HPLC. Catechins were the most important class in mango and tamarind jams (36.93% and 40.53%, respectively), while phenolic acids (cinnamic acids plus benzoic acids) were the most important classes in guava jelly (67.75%) and in lychee (86.46%), orange (76.77%) and red guava (54.82%) jams. Banana flower showed a high percentage of tannins and anthocyanins (27.21% and 29.42%, respectively), followed by catechins (16.81%) and benzoic acids (14.53%).
Analysed jams and jellies presented interesting quali-quantitative polyphenolic profiles if compared to commercial products derived from common temperate fruits; in particular, they showed higher TPC values and relative antioxidant activity than strawberry (101.40 mg
GAE/100 g
Pr) [
25], apricot (51.49 mg
GAE/100 g
Pr) [
21], berry fruits (336.67 mg
GAE/100 g
Pr) [
24], peach (18.85 mg
GAE/100 g
Pr) and apple (20.07 mg
GAE/100 g
Pr) [
16]. Moreover, jams from tropical fruits showed higher values of specific phenolic markers as coumaric, caffeic and ferulic acids (lychee, banana and guava), catechin (tamarind) and rutin (mango) than commercial products derived from berry fruits (the content values in berry fruits were 0.39 mg/100 g
Pr for coumaric acid, 1.38 mg/100 g
Pr for caffeic acid, 0.13 mg/100 g
Pr for ferulic acid, 3.93 mg/100 g
Pr for catechin and 0.26 mg/100 g
Pr for rutin) [
19]. These results may contribute to better valorise the products derived from local biodiversity compared to imported commercial ones and improve food industry in the Comoros Islands with the potential exportation of these productions.
In some studies, phenolic compound characterisation was mainly used for chemotaxonomic purposes; moreover, some researchers reported that fruit processing during jam/jelly production did not change much the qualitative polyphenolic profile [
10,
30]. In this research, each product showed a specific phenolic composition characterised by the presence of one or more specific markers. Chlorogenic acid proved to be characteristic of tamarind jam (11.45 ± 0.73 mg/100 g
Pr), guava jelly (13.77 ± 0.55 mg/100 g
Pr) and orange jam (12.97 ± 0.19 mg/100 g
Pr) and it could be used as a marker to prove the addition of these fruits to other jams. Moreover, chlorogenic acid is considered a preferential substrate for the catecholase activity of polyphenol oxidase and it may be important during fruit processing [
31]. Caffeic acid was detected in all the fruit-derived products in a close range between 0.49 ± 0.13 mg/100 g
Pr (tamarind jam, CM3) and 0.76 ± 0.12 mg/100 g
Pr (lychee jam, CM6) similar to other studies [
32,
33]. Coumaric acid was the most important cinnamic acid in red guava jam-CM8 (5.58 ± 0.80 mg/100 g
Pr), while ferulic acid was present in tamarind jam-CM3 (1.38 ± 0.22 mg/100 g
Pr) and orange jam-CM7 (1.00 ± 0.11 mg/100 g
Pr) as reported by Jimohand Onabanjo [
34] and Marquina et al. [
35]. Caffeic, ferulic and coumaric acids could be involved in the oxidation processes and colour development during technological processing [
36]. Flavonoids (flavanols and catechins) were also demonstrated to be important as markers for orange and tamarind jam quality control [
37], because flavonoids are not affected by the manufacturing process. Flavonols quench active oxygen species [
38] and inhibit in vitro oxidation of low-density lipoproteins reducing thrombotic tendency. In this research, the most effective flavonol selected as marker was quercetin in guava jelly (7.56 ± 0.03 mg/100 g
Pr) and jams of tamarind (11.44 ± 0.96 mg/100 g
Pr), orange (15.69 ± 1.11 mg/100 g
Pr) and red guava (7.62 ± 0.20 mg/100 g
Pr), while rutin proved to be characteristic of mango jams (0.94 ± 0.04 mg/100 g
Pr for CM1 and 0.86 ± 0.03 mg/100 g
Pr for CM2) according to other studies [
39,
40]. The identification of catechin (maximum value of 40.64 ± 1.56 mg/100 g
Pr in tamarind jam) and epicatechin (maximum value of 4.03 ± 0.16 mg/100 g
Pr in red guava jam) could be useful: indeed, they are involved in the lipid peroxidation inhibition and human cancer cell line proliferation as other similar compounds [
41]. The presence of tannins in adequate amounts in orange and red guava jams (18.24 ± 2.52 mg/100 g
Pr and 19.79 ± 0.50 mg/100 g
Pr, respectively) are positive as they are free radical quenchers [
42]. High levels of ellagic acid in tamarind jam (23.30 ± 1.96 mg/100 g
Pr) and orange jam (57.11 ± 1.64 mg/100 g
Pr) as well as high content of gallic acid in guava jelly (42.02 ± 0.58 mg/100 g
Pr) and orange jam (87.72 ± 1.81 mg/100 g
Pr) were also detected: these molecules are endowed with numerous biological properties, as anticancer, anti-inflammatory and anti-HIV replication activities [
43]. These preliminary results on phenolic composition demonstrate the need of identifying more bioactive substances for control of the authenticity of fruit-based products.
Anthocyanins have frequently been also considered for the cited purposes, because their specific patterns may allow the classification of fruit species and relative derived products and the characterization of their nutraceutical and nutritional traits (e.g., detection of admixtures of fruits with a more stable colour during jam processing). As previously discussed, anthocyanins are of prominent importance in guava jelly (8.35 ± 1.13 mg
C3G/100g
Pr), orange jam (9.56 ± 0.46 mg
C3G/100g
Pr) and red guava jam (4.99 ± 0.67 mg
C3G/100g
Pr) because i) they are important for quality traits, due to their levels directly related to the product colour and ii) they have been proved to show several health-promoting activities and a high potential phytochemical value [
44]. As opposed to other polyphenolic compounds, composition in anthocyanins may be subject to modification during processing and storage steps as reported by Garzon and Wrolstad [
45]: in particular, enzymation during jam processing may change anthocyanin patterns [
46]. During jam and jelly storage new pyranoanthocyanins may also be formed by direct reaction of anthocyanins with cinnamic acids, as shown by Schwarz et al. [
47]. For this reason, anthocyanins may be only used as quantitative markers in the quality control of jams and similar products.
Monoterpenes represent an important fraction of the TBCC in analysed fruit-based products, in particular limonene in mango jam (103.53 ± 3.12 mg/100 g
Pr) and guava jelly (102.75 ± 0.58 mg/100 g
Pr) and γ-terpinene in jams of mango (48.07 ± 2.30 mg/100 g
Pr) and red guava (43.01 ± 0.16 mg/100 g
Pr): the plant terpenoids are a large class of phytochemicals used for their aromatic qualities and antioxidant and anti-inflammatory activity [
48]. Monoterpenes are non-nutritive dietary substances with antibacterial and antitumor activity found in the essential oils of several plants [
49] and several studies reported their chemopreventive activity against several cancers [
50].
Vitamin C value was obtained as the sum of ascorbic and dehydroascorbic acids due to their biological activity in humans [
51]. In this research banana flower and orange jams showed a high vitamin C content (52.82 ± 0.57 mg/100 g
Pr and 86.07 ± 0.48 mg/100 g
Pr, respectively). Other analysed products showed good values of vitamin C (about 10–25 mg/100 g
Pr) according to previous similar studies [
52,
53].
Organic acids in fruits are little influenced by changes during processing and storage and show a good stability if compared to pigments and flavour compounds. Accordingly, their identification may be suitable for the estimation of fruit amount and for the fruit quality control [
54]. However, since organic acids (e.g., citric acid) are indispensable technological components of most derived-products, they are not applicable as quality control markers in fruit jams and jellies. Furthermore, organic acid composition is influenced by genetic factors (e.g., cultivar) and degree of ripeness, limiting their applicability as a quantitative marker in fruit-derived products [
55]. In any case, they are important antioxidants with multi-purpose uses in pharmacology as reported by Eyduran et al. [
56]. They were also utilised as food acidifiers by food companies [
57]. In this research orange jam showed high variability in organic acid composition: in particular, succinic acid (406.65 ± 4.40 mg/100 g
Pr) and quinic acid (391.13 ± 2.19 mg/100 g
Pr) were found to be suitable markers for jam characterization because they were not detected in other analysed fruit-based products and represent specific molecules of orange jam chromatographic pattern as shown by Cejudo-Bastante et al. [
58] and Flores et al. [
59]. Similarly, malic acid could be a specific marker for mango jams and tartaric acid for guava jelly and jams of lychee and red guava.
Figure 3 shows total organic acid content in analysed fruit-based products.
Apart from organic acids, the sugar pattern was also utilised for fruit species differentiation, while their use as quantifiers of fruit content is very limited. Identical sugar profiles, used often for the detection of illegal adulterations as the admixture of sugar solutions or fruit juices, were observed for fruits from different countries as well as for different genotype [
55]. In this research sugar pattern (
Figure 4) was studied in order to evaluate the nutritional potential of analysed fruit-derived products. Tamarind jam showed the highest sugar content (56.50 ± 2.70 g/100 g
Pr), expressed as sum of fructose (15.35 ± 0.14 g/100 g
Pr), glucose (27.64 ± 2.33 g/100 g
Pr) and sucrose (13.51 ± 0.23 g/100 g
Pr). Other analysed products presented a total sugar content of about 40 g/100 g
Pr as reported in other studies [
60,
61].