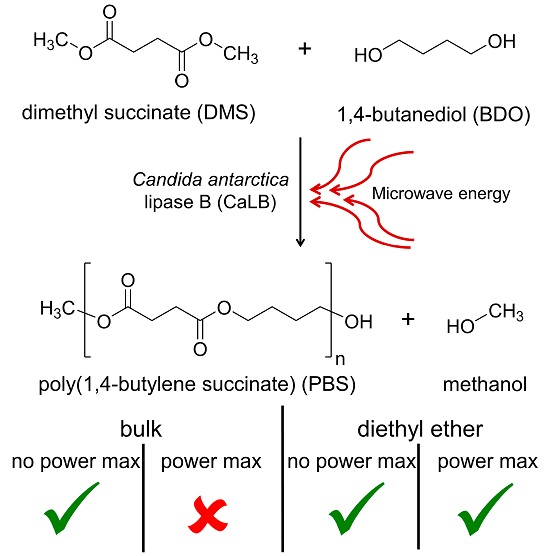
On the Effect of Microwave Energy on Lipase-Catalyzed Polycondensation Reactions
Abstract
:1. Introduction
- Microwave energy can be introduced remotely without the need for direct contact between the energy source and the chemicals, thus avoiding hot spots and improving product selectivity
- Energy input starts and stops immediately when power is turned on or off, thus when hazardous exotherms are encountered the MW irradiation can be instantly removed
- Heating rates are high as long as one of the components of the reaction can couple strongly with the microwaves
- Energy consumption is generally considerably lower for microwave assisted reactions as the reaction media or reagents are heated directly without prior need of heating the reaction vessel and surrounding equipment
2. Materials and Methods
2.1. Materials and Enzymes
2.2. Polycondensation Reactions
2.2.1. Solvent-Free Reactions
2.2.2. Reactions in Organic Media
2.3. 1H-NMR Spectroscopy Analysis
2.4. Gel Permeation Chromatography (GPC)
3. Results and Discussion
3.1. Solvent-Free Reactions
3.2. Reactions in Organic Media
4. Conclusions
Supplementary Materials
Acknowledgments
Access Statement
Author Contributions
Conflicts of Interest
Abbreviations
| MWe | microwave energy |
| CaLB | Candida antarctica lipase B |
| ROP | ring opening polymerization |
| ε-CL | ε-caprolactone |
| DMA | dimethyl adipate |
| DMS | dimethyl succinate |
| BDO | 1,4-butanediol |
References
- Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Sherwood, J. Circular economy design considerations for research and process development in the chemical sciences. Green Chem. 2016, 18, 3914–3934. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Ferrario, V.; Zartl, B.; Brandauer, M.; Gamerith, C.; Herrero Acero, E.; Ebert, C.; Gardossi, L.; Guebitz, G.M. Enlarging the tools for efficient enzymatic polycondensation: Structural and catalytic features of cutinase 1 from Thermobifida cellulosilytica. Catal. Sci. Technol. 2016, 6, 3430–3442. [Google Scholar] [CrossRef]
- Feder, D.; Gross, R.A. Exploring Chain Length Selectivity in HIC-Catalyzed Polycondensation Reactions. Biomacromolecules 2010, 11, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Guarneri, A.; Brandauer, M.; Herrero Acero, E.; Peerlings, H.; Gardossi, L.; Guebitz, G.M. Exploring mild enzymatic sustainable routes for the synthesis of bio-degradable aromatic-aliphatic oligoesters. Biotechnol. J. 2016, 11, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Corici, L.; Pellis, A.; Ferrario, V.; Ebert, C.; Cantone, S.; Gardossi, L. Understanding Potentials and Restrictions of Solvent-Free Enzymatic Polycondensation of Itaconic Acid: An Experimental and Computational Analysis. Adv. Synth. Catal. 2015, 357, 1763–1774. [Google Scholar] [CrossRef]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J.; Ogunjobi, J. Post-polymerisation Modification of Bio-derived Unsaturated Polyester Resins via Michael Additions of 1,3-dicarbonyls. Polym. Chem. 2016, 7, 1650–1658. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of Unsaturated Polyester Resins from Various Bio-Derived Platform Molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bansode, S.R.; Ribeiro, A.; Abreu, A.S.; Oliveira, C.; Parpot, P.; Gogate, P.R.; Rathod, V.K.; Cavaco-Paulo, A. Ultrasound enhances lipase-catalyzed synthesis of poly (ethylene glutarate). Ultrason. Sonochem. 2016, 31, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.M.; Annuar, M.S.M.; Chisti, Y.; Heidelberg, T. Ultrasound assisted lipase catalyzed synthesis of poly-6-hydroxyhexanoate. Ultrason. Sonochem. 2012, 19, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Rejasse, B.; Lamare, S.; Legoy, M.-D.; Besson, T. Influence of microwave irradiation on enzymatic properties: Applications in enzyme chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 519–527. [Google Scholar] [CrossRef]
- Mahapatro, A.; Matos Negrón, T.D. Microwave-Assisted Biocatalytic Polymerizations. Green Polym. Chem. 2013, 1144, 69–80. [Google Scholar]
- Strauss, C.R. Applications of Microwaves for Environmentally Benign Organic Chemistry. Handb. Green Chem. Technol. 2002, 397–415. [Google Scholar]
- Roberts, B.A.; Strauss, C.R. Toward rapid, “green”, predictable microwave-assisted synthesis. Acc. Chem. Res. 2005, 38, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.S.P.; Jothilingam, S. Solvent-free microwave-mediated Michael addition reactions. J. Chem. Sci. 2005, 117, 323–328. [Google Scholar] [CrossRef]
- Gronnow, M.J.; White, R.J.; Clark, J.H.; Macquarrie, D.J. Energy efficiency in chemical reactions: A comparative study of different reaction techniques. Org. Proc. Res. Dev. 2005, 9, 516–518. [Google Scholar] [CrossRef]
- Rouillard, H.; Deau, E.; Domon, L.; Chérouvrier, J.-R.; Graber, M.; Thiéry, V. Microwave-assisted kinetic resolution of homochiral (Z)-cyclooct-5-ene-1,2-diol and (Z)-2-acetoxycyclooct-4-enyl acetate using lipases. Molecules 2014, 19, 9215–9227. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.M.; Carretoni, C.; Cruz, R.; Freitas, S.; Melo, P.A., Jr.; Costa-Félix, R.; Pinto, J.C.; Nele, M. Microwave activation of enzymatic catalysts for biodiesel production. J. Mol. Catal. B 2010, 67, 117–121. [Google Scholar] [CrossRef]
- Réjasse, B.; Lamare, S.; Legoy, M.-D.; Besson, T. Stability improvement of immobilized Candida antarctica lipase B in an organic medium under microwave radiation. Org. Biomol. Chem. 2004, 2, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Réjasse, B.; Besson, T.; Legoy, M.-D.; Lamare, S. Influence of microwave radiation on free Candida antarctica lipase B activity and stability. Org. Biomol. Chem. 2006, 4, 3703–3707. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.-C.; Besson, T.; Lamare, S.; Legoy, M.-D. Microwave radiation can increase the rate of enzyme-catalysed reactions in organic media. Tetrahedron Lett. 1996, 37, 8383–8386. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Ni, Y.; Zheng, G.-W.; Chen, H.-H.; Zhu, Z.-R.; Xu, J.-H. Enzymatic resolution of a chiral chlorohydrin precursor for (R)-α-lipoic acid synthesis via lipase catalyzed enantioselective transacylation with vinyl acetate. J. Mol. Catal. B 2014, 99, 102–107. [Google Scholar] [CrossRef]
- Bradoo, S.; Rathi, P.; Saxena, R.K.; Gupta, R. Microwave-assisted rapid characterization of lipase selectivities. J. Biochem. Biophys. Methods 2002, 51, 115–120. [Google Scholar] [CrossRef]
- Kerep, P.; Ritter, H. Influence of Microwave Irradiation on the Lipase-Catalyzed Ring-Opening Polymerization of ε-Caprolactone. Macromol. Rapid Commun. 2006, 27, 707–710. [Google Scholar] [CrossRef]
- Kerep, P.; Ritter, H. Chemoenzymatic Synthesis of Polycaprolactone-block-Polystyrene via Macromolecular Chain Transfer Reagents. Macromol. Rapid Commun. 2007, 28, 759–766. [Google Scholar] [CrossRef]
- Matos, T.D.; King, N.; Simmons, L.; Walker, C.; McClain, A.R.; Mahapatro, A.; Rispoli, F.J.; McDonnell, K.T.; Shah, V. Microwave assisted lipase catalyzed solvent-free poly-ε-caprolactone synthesis. Green Chem. Lett. Rev. 2011, 4, 73–79. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The potential of microwave technology for the recovery, synthesis and manufacturing of chemicals from bio-wastes. Catal. Today 2015, 239, 80–89. [Google Scholar] [CrossRef]
- Breeden, S.W.; Clark, J.H.; Farmer, T.J.; Macquarrie, D.J.; Meimoun, J.S.; Nonne, Y.; Reid, J. Microwave heating for rapid conversion of sugars and polysaccharides to 5-chloromethyl furfural. Green Chem. 2013, 15, 72–75. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Farmer, T.J.; Mascal, M. Chapter 4: Platform molecules. In Introduction to Chemicals from Biomass; John Wiley & Sons Ltd.: Chichester, UK, 2014. [Google Scholar]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Lee, S.-J.; Park, J.-W.; Kim, H.-J. Synthesis and properties of flexible polyester with urethane polyol for automotive pre-coated metals. J. Ades. Sci. Technol. 2016, 30, 1537–1554. [Google Scholar] [CrossRef]
- Vouyiouka, S.; Topakas, E.; Katsini, A.; Papaspyrides, C.; Christakopoulos, P. A green route for the preparation of aliphatic polyesters via lipase-catalyzed prepolymerization and low-temperature post polymerization. Macromol. Mater. Eng. 2013, 298, 679–689. [Google Scholar] [CrossRef]
- Theiler, S.; Diamantouros, S.E.; Jockenhoevel, S.; Keul, H.; Moeller, M. Synthesis and characterization of biodegradable polyester/polyether resins via Michael-type addition. Polym. Chem. 2011, 2, 2273–2283. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.





| Entry (No.) | Diester (A) | Diol (B) | Power (W) | T (°C) | Vessel | Difference vs. Oil Bath | ||
|---|---|---|---|---|---|---|---|---|
| Set Max | Power Max | Open/Close | Conversion (%) * | Mw (Da) λ | ||||
| 1 | DMA | BDO | 200 | - | 50 | Close | = | = |
| 2 | DMS | BDO | 200 | - | 50 | Close | = | = |
| 3 | DMS | BDO | 100 | - | 50 | Close | = | = |
| 4 | DMS | BDO | 200 | - | 50 | Open | = | −173 |
| 5 | DMA | BDO | 100 | - | 50 | Open | = | −125 |
| 6 | DMS | BDO | 200 | - | 70 | Open | = | = |
| 7 | DMS | BDO | 200 | + | 50 | Open | −75 | −220 |
| 8 | DMS | BDO | 200 | + | 70 | Open | −60 | −114 |
| Entry (No.) | Diester (A) | Diol (B) | Power (W) | T (°C) | Difference vs. Oil Bath | ||
|---|---|---|---|---|---|---|---|
| Set Max | Power Max | Conversion (%) * | Mw (Da) λ | ||||
| 9 | DMS | BDO | 200 | - | 30 | = | = |
| 10 | DMA | BDO | 200 | - | 30 | = | = |
| 11 | DMS | BDO | 200 | - | 38 | = | = |
| 12 | DMS | BDO | 100 | + | 38 | = | = |
| 13 | DMS | BDO | 200 | + | 38 | = | = |
| Entry (No.) | Reaction Type | Power Max | T (°C) | Time (min) | Av. Power (W/s) | Conversion (%) * |
|---|---|---|---|---|---|---|
| 11a | Diethyl ether | No | 38 | 60 | 0.02 | 45 |
| 13a | Diethyl ether | Yes | 38 | 60 | 21.58 | 47 |
| 4a | Bulk | No | 50 | 60 | 1.01 | 47 |
| 7a | Bulk | Yes | 50 | 60 | 64.49 | 11 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellis, A.; Guebitz, G.M.; Farmer, T.J. On the Effect of Microwave Energy on Lipase-Catalyzed Polycondensation Reactions. Molecules 2016, 21, 1245. https://doi.org/10.3390/molecules21091245
Pellis A, Guebitz GM, Farmer TJ. On the Effect of Microwave Energy on Lipase-Catalyzed Polycondensation Reactions. Molecules. 2016; 21(9):1245. https://doi.org/10.3390/molecules21091245
Chicago/Turabian StylePellis, Alessandro, Georg M. Guebitz, and Thomas J. Farmer. 2016. "On the Effect of Microwave Energy on Lipase-Catalyzed Polycondensation Reactions" Molecules 21, no. 9: 1245. https://doi.org/10.3390/molecules21091245