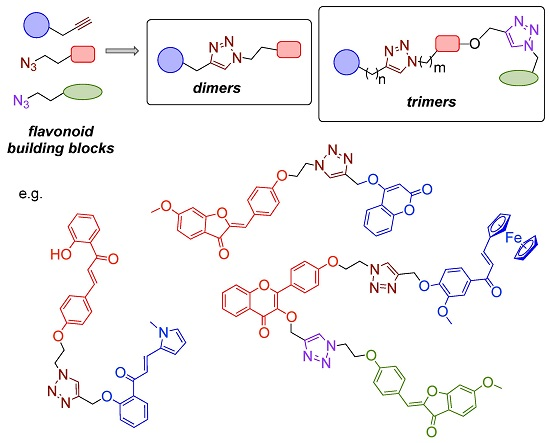
3.3. Synthesis of Triazole-Bridged Flavonoid Dimers and Trimers
(E)-3-(3,4-Dimethoxyphenyl)-1-(3-methoxy-4-(2-(4-((2-methoxy-4-((E)-3-(4-methoxyphenyl)acryloyl))methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)prop-2-en-1-one (90). A mixture of alkyne chalcone 20 (254 mg, 0.789 mmol), azide chalcone 67 (305 mg, 0.796 mmol), CuSO4·5H2O (225 mg, 0.900 mmol) and sodium ascorbate (389 mg, 1.97 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 90 (151 mg, 27%) as a pale yellow-brown powdery solid. m.p. 108–110 °C. TLC Rf = 0.41 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2942w (C-H str), 2837w (C-H str), 1652m (C=O str), 1595m (C=C str), 1572m (C=C str), 1509s (C=C str), 1464m, 1420m, 1335w, 1307w, 1255s, 1195w, 1143s, 1021s. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.92 (3H, s, -OCH3), 3.94 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 3.96 (3H, s, -OCH3), 4.46 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.40 (2H, s, -OCH2CN-), 6.82 (1H, d, J = 8.0 Hz, ArH), 6.90 (1H, d, J = 8.4 Hz, ArH), 6.93 (2H, d, J = 8.8 Hz, ArH), 7.14–7.17 (2H, m, ArH), 7.24 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.37 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.41 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.57–7.62 (6H, m, ArH), 7.75 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.77 (1H, d, J = 15.6 Hz, -CH=CHCO-), 8.04 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.4, 56.0, 56.0, 62.9, 67.6, 110.2, 111.1, 111.1, 111.5, 112.3, 112.4, 114.4, 119.2, 119.4, 122.5, 122.6, 123.0, 124.7, 127.7, 127.9, 130.1, 132.1, 132.8, 143.7, 144.0, 144.5, 149.2, 149.5, 149.7, 151.2, 151.4, 151.6, 161.5, 188.6. LCMS (ES+) m/z = 706.2 ([M + H]+, tR = 1.65 min). HRMS (ESI+) m/z = 728.2576 [M + Na]+ found, C40H39O9N3Na+ required 728.2579.
(E)-1-(3-Methoxy-4-((1-(2-(2-methoxy-4-((E)-3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (91). A mixture of alkyne chalcone 20 (234 mg, 0.727 mmol), azide chalcone 69 (303 mg, 0.732 mmol), CuSO4·5H2O (203 mg, 0.813 mmol) and sodium ascorbate (361 mg, 1.82 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 91 (250 mg, 47%) as a pale yellow-white flaky solid. m.p. 206–208 °C. TLC Rf = 0.44 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3150w (C-H str), 2942w (C-H str), 1661m (C=O str), 1601s (C=C str), 1572s (C=C str), 1514s (C=C str), 1459m, 1416m, 1324s, 1295m, 1262s, 1228m, 1166s, 1150s, 1126s, 1053w, 1015s. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.87 (3H, s, -OCH3), 3.91 (6H, s, 2 × -OCH3), 3.92 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 4.46 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.40 (2H, s, -OCH2CN-), 6.15 (2H, s, ArH), 6.85 (1H, d, J = 8.4 Hz, ArH), 6.94 (2H, d, J = 8.8 Hz, ArH), 7.17 (1H, d, J = 8.8 Hz, ArH), 7.42 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.58–7.65 (6H, m, ArH), 7.78 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.85 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.05 (1H, s, -CHN-), 8.23 (1H, d, J = 16.0 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.4, 55.8, 55.9, 56.0, 62.9, 67.5, 90.5, 106.6, 111.1, 111.7, 112.3, 112.4, 114.4, 119.3, 121.6, 122.3, 122.6, 124.7, 127.8, 130.1, 132.1, 133.7, 135.6, 143.7, 143.9, 149.5, 149.5, 150.6, 151.6, 161.5, 161.7, 163.0, 188.6, 190.3. LCMS (ES+) m/z = 736.2 ([M + H]+, tR = 1.67 min). HRMS (ESI+) m/z = 736.2892 [M + H]+ found, C41H42O10N3+ required 736.2865.
(E)-1-(2-Hydroxy-4-methoxyphenyl)-3-(4-methoxy-3-((1-(2-(2-methoxy-4-((E)-3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one (92). A mixture of alkyne chalcone 4 (167 mg, 0.494 mmol), azide chalcone 69 (206 mg, 0.498 mmol), CuSO4·5H2O (140 mg, 0.560 mmol) and sodium ascorbate (253 mg, 1.28 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 92 (236 mg, 64%) as a bright yellow flaky solid. m.p. 208–210 °C. TLC Rf = 0.48 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2931w (C-H str), 2842w (C-H str), 1632m (C=O str), 1597m (C=C str), 1568s (C=C str), 1509s (C=C str), 1455w, 1419w, 1371m, 1318m, 1259s, 1232s, 1211s, 1153s, 1123s, 1021m, 1004m. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.87 (3H, s, -OCH3), 3.91 (3H, s, -OCH3), 3.91 (9H, s, 3 × -OCH3), 4.45 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.38 (2H, s, -OCH2CN-), 6.14 (2H, s, ArH), 6.46 (1H, d, J = 2.4 Hz, ArH), 6.50 (1H, dd, J = 8.8, 2.4 Hz, ArH), 6.81 (1H, d, J = 8.4 Hz, ArH), 6.89 (1H, d, J = 2.4 Hz, ArH), 7.21 (1H, dd, J = 8.0, 2.0 Hz, ArH), 7.45 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.46 (1H, d, J = 1.6 Hz, ArH), 7.55 (1H, dd, J = 8.4, 1.6 Hz, ArH), 7.61 (1H, d, J = 1.6 Hz, ArH), 7.77 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.85 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.89 (1H, d, J = 8.8 Hz, ArH), 8.05 (1H, s, -CHN-), 8.23 (1H, d, J = 16.0 Hz, -CH=CHCO-), 13.55 (1H, s, OH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.4, 55.5, 55.8, 55.9, 56.0, 63.2, 67.6, 90.5, 101.0, 106.6, 107.6, 111.5, 111.6, 112.3, 113.0, 114.2, 118.4, 121.5, 122.3, 124.3, 124.7, 127.8, 131.3, 133.7, 135.6, 144.0, 144.1, 147.8, 149.4, 150.6, 152.0, 161.7, 163.0, 166.0, 166.6, 190.3, 191.8. LCMS (ES+) m/z = 752.2 ([M + H]+, tR = 1.74 min). HRMS (ESI+) m/z = 752.2822 [M + H]+ found, C41H42O11N3+ required 752.2814.
(E)-1-(3-Methoxy-4-((1-(2-(2-methoxy-4-((E)-3-(2,3,4-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (93). A mixture of alkyne chalcone 20 (235 mg, 0.729 mmol), azide chalcone 68 (303 mg, 0.733 mmol), CuSO4·5H2O (271 mg, 1.09 mmol) and sodium ascorbate (414 mg, 2.09 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 93 (515 mg, 96%) as a pale yellow-green flaky solid. m.p. 128–130 °C. TLC Rf = 0.30 (4% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2937w (C-H str), 2836w (C-H str), 1652m (C=O str), 1595s (C=C str), 1572s (C=C str), 1510s (C=C str), 1494s, 1463s, 1415s, 1326w, 1255s, 1193w, 1148s, 1095s, 1021s. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.90 (3H, s, -OCH3), 3.92 (6H, s, 2 × -OCH3), 3.95 (6H, s, 2 × -OCH3), 4.47 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.40 (2H, s, -OCH2CN-), 6.73 (1H, d, J = 8.8 Hz, ArH), 6.84 (1H, d, J = 8.4 Hz, ArH), 6.94 (2H, d, J = 8.8 Hz, ArH), 7.17 (1H, d, J = 8.8 Hz, ArH), 7.39 (1H, d, J = 8.8 Hz, ArH), 7.42 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.54 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.59–7.64 (6H, m, ArH), 7.78 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.98 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.05 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.4, 56.0, 56.0, 56.1, 60.9, 61.4, 62.9, 67.5, 107.6, 111.1, 111.6, 112.3, 112.4, 114.4, 119.2, 120.8, 122.0, 122.5, 122.6, 123.9, 124.7, 127.7, 130.1, 132.1, 133.0, 139.7, 142.5, 143.7, 144.0, 149.5, 149.7, 151.1, 151.6, 153.8, 155.7, 161.5, 188.6, 189.0. LCMS (ES+) m/z = 736.2 ([M + H]+, tR = 1.66 min). HRMS (ESI+) m/z = 736.2855 [M + H]+ found, C41H42O10N3+ required 736.2870.
(E)-1-(3-Methoxy-4-((1-(2-(2-methoxy-4-((E)-3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(1-methyl-1H-pyrrol-2-yl)prop-2-en-1-one (94). A mixture of alkyne chalcone 22 (217 mg, 0.734 mmol), azide chalcone 69 (305 mg, 0.737 mmol), CuSO4·5H2O (214 mg, 0.857 mmol) and sodium ascorbate (365 mg, 1.84 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 94 (367 mg, 70%) as a bright yellow flaky solid. m.p. 138–140 °C. TLC Rf = 0.31 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2838w (C-H str), 1646m (C=O str), 1596m (C=C str), 1566s (C=C str), 1512m (C=C str), 1470m, 1457m, 1414m, 1338w, 1321m, 1257s, 1198m, 1155s, 1146s, 1118s, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.77 (3H, s, -NCH3), 3.86 (3H, s, -OCH3), 3.91 (6H, s, 2 × -OCH3), 3.91 (3H, s, -OCH3), 3.94 (3H, s, -OCH3), 4.45 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.83 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.38 (2H, s, -OCH2CN-), 6.14 (2H, s, ArH), 6.21–6.23 (1H, m, ArH), 6.80–6.86 (3H, m, ArH), 7.16 (1H, d, J = 8.8 Hz, ArH), 7.29 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.57–7.63 (4H, m, ArH), 7.79 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.85 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.05 (1H, s, -CHN-), 8.23 (1H, d, J = 15.6 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 34.4, 49.7, 55.4, 55.8, 55.9, 56.0, 62.8, 67.5, 90.5, 106.5, 109.6, 111.1, 111.7, 112.1, 112.3, 112.4, 116.3, 121.5, 122.3, 122.3, 124.7, 127.6, 130.3, 131.6, 132.4, 133.7, 135.6, 143.7, 149.4, 149.5, 150.6, 151.4, 161.6, 163.0, 188.1, 190.3. LCMS (ES+) m/z = 709.3 ([M + H]+, tR = 1.63 min). HRMS (ESI+) m/z = 709.2854 [M + H]+ found, C39H41O9N4+ required 709.2868.
(E)-1-(3-Methoxy-4-((1-(2-(2-methoxy-4-((E)-3-(1-methyl-1H-pyrrol-2-yl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(1-methyl-1H-pyrrol-2-yl)prop-2-en-1-one (95). A mixture of alkyne chalcone 22 (271 mg, 0.918 mmol), azide chalcone 70 (301 mg, 0.924 mmol), CuSO4·5H2O (316 g, 1.26 mmol) and sodium ascorbate (499 mg, 2.52 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 95 (555 mg, 97%) as a dark yellow-brown flaky solid. m.p. 118–120 °C. TLC Rf = 0.38 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2844w (C-H str), 1644m (C=O str), 1594m (C=C str), 1564s (C=C str), 1511m (C=C str), 1480m, 1412m, 1381w, 1330m, 1258s, 1196m, 1153s, 1129m, 1055w, 1025m. 1H-NMR (500 MHz, CDCl3): δ 3.76 (3H, s, -NCH3), 3.77 (3H, s, -NCH3), 3.92 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 4.46 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.39 (2H, s, -OCH2CN-), 6.22–6.24 (2H, m, ArH), 6.82–6.86 (5H, m, ArH), 7.15 (1H, d, J = 8.0 Hz, ArH), 7.27 (1H, d, J = 15.2 Hz, -CH=CHCO-, overlain by CDCl3), 7.29 (1H, d, J = 14.0 Hz, -CH=CHCO-), 7.57–7.62 (4H, m, ArH), 7.79 (2H, d, J = 14.8 Hz, 2 × -CH=CHCO-), 8.04 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 34.4, 49.7, 55.9, 56.0, 62.9, 67.5, 109.6, 109.7, 111.1, 111.4, 112.1, 112.2, 112.3, 112.4, 116.2, 116.3, 122.1, 122.3, 124.7, 127.6, 127.7, 130.3, 130.3, 131.6, 131.8, 132.4, 133.1, 143.7, 149.4, 149.6, 150.9, 151.4, 188.0, 188.1. LCMS (ES+) m/z = 622.3 ([M + H]+, tR = 1.62 min). HRMS (ESI+) m/z = 622.2648 [M + H]+ found, C35H36O6N5+ required 622.2660.
(E)-1-(2-Hydroxyphenyl)-3-(4-(2-(4-((2-((E)-3-(1-methyl-1H-pyrrol-2-yl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)prop-2-en-1-one (96). A mixture of alkyne chalcone 23 (337 mg, 1.27 mmol), azide chalcone 61 (400 mg, 1.29 mmol), CuSO4·5H2O (429 mg, 1.72 mmol) and sodium ascorbate (641 mg, 3.23 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 96 (399 mg, 55%) as a bright yellow-orange powdery solid. m.p. 98–100 °C. TLC Rf = 0.40 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2933w (C-H str), 2884w (C-H str), 1635m (C=O str), 1597m (C=C str), 1562s (C=C str), 1509m (C=C str), 1480m, 1447m, 1329m, 1286m, 1268m, 1233m, 1202s, 1174s, 1156s, 1113w, 1055m, 1025s. 1H-NMR (500 MHz, CDCl3): δ 3.65 (3H, s, -NCH3), 4.32 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.65 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.33 (2H, s, -OCH2CN-), 6.16–6.18 (1H, m, ArH), 6.63 (1H, dd, J = 4.0, 1.2 Hz, ArH), 6.76 (1H, t, J = 2.0 Hz, ArH), 6.85 (2H, d, J = 8.8 Hz, ArH), 6.95 (1H, t, J = 8.0 Hz, ArH), 7.03 (1H, dd, J = 8.4, 0.8 Hz, ArH), 7.08 (1H, d, J = 7.6 Hz, ArH), 7.12 (1H, d, J = 8.4 Hz, ArH), 7.16 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.44–7.59 (4H, m, ArH), 7.54 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.60 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.66 (1H, dd, J = 8.0, 2.0 Hz, ArH), 7.76 (1H, s, -CHN-), 7.86 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.93 (1H, dd, J = 8.4, 1.6 Hz, ArH), 12.89 (1H, s, OH). 13C-NMR (500 MHz, CDCl3): δ 34.3, 49.5, 63.0, 66.2, 109.6, 112.4, 113.0, 114.9, 118.2, 118.5, 118.8, 120.0, 121.4, 122.2, 123.9, 127.7, 128.2, 129.5, 130.0, 130.1, 130.5, 130.6, 132.7, 136.2, 144.1, 144.8, 156.5, 159.9, 163.5, 191.9, 193.5. LCMS (ES+) m/z = 575.3 ([M + H]+, tR = 1.72 min). HRMS (ESI+) m/z = 575.2266 [M + H]+ found, C34H31O5N4+ required 575.2289.
(E)-3-(Ferrocenyl)-1-(4-((1-(2-(4-((E)-3-(2-hydroxyphenyl)-3-oxoprop-1-en-1-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxyphenyl)prop-2-en-1-one (97). A mixture of alkyne chalcone 25 (301 mg, 0.753 mmol), azide chalcone 61 (235 mg, 0.760 mmol), CuSO4·5H2O (225 mg, 0.900 mmol) and sodium ascorbate (398 mg, 2.01 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 97 (427 mg, 80%) as a dark red-brown powdery solid. m.p. 178–180 °C. TLC Rf = 0.28 (2% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2937w (C-H str), 1740m (C=O str), 1651m, 1637s, 1596s (C=C str), 1571s (C=C str), 1510s (C=C str), 1487m, 1444m, 1341w, 1300m, 1266s, 1203s, 1179s, 1159s, 1146s, 1048m, 1020s. 1H-NMR (500 MHz, CDCl3): δ 3.98 (3H, s, -OCH3), 4.16 (5H, s, -C5H5), 4.42 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.47 (2H, t, J = 2.0 Hz, -C5H4), 4.56 (2H, t, J = 2.0 Hz, -C5H4), 4.80 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.43 (2H, s, -OCH2CN-), 6.84 (2H, d, J = 8.8 Hz, ArH), 6.97 (1H, t, J = 8.0 Hz, ArH), 7.04 (1H, dd, J = 8.4, 0.8 Hz, ArH), 7.12 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.12 (1H, d, J = 8.4 Hz, ArH), 7.51 (1H, t, J = 8.4 Hz, ArH), 7.54–7.61 (3H, m, ArH), 7.59 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.64 (1H, d, J = 1.6 Hz, ArH), 7.74 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.86 (1H, s, -CHN-), 7.86 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.97 (1H, dd, J = 8.0, 1.6 Hz, ArH), 12.90 (1H, s, OH). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.1, 62.8, 66.3, 68.9, 69.7, 71.3, 79.2, 111.1, 112.3, 114.9, 118.3, 118.5, 118.6, 118.8, 120.0, 122.4, 124.3, 128.4, 129.6, 130.6, 132.3, 136.3, 143.8, 144.7, 146.1, 149.5, 151.2, 159.8, 163.6, 187.9, 193.6. LCMS (ES+) m/z = 710.2 ([M + H]+, tR = 2.09 min). HRMS (ESI+) m/z = 710.1889 [M + H]+ found, C40H36N3O6Fe+ required 710.1875.
(E)-3-((1-(2-(4-(3-(3,4-Dimethoxyphenyl)acryloyl)-2-methoxyphenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (98). A mixture of alkyne flavone 30 (806 mg, 2.92 mmol), azide chalcone 67 (1.01 g, 2.64 mmol), CuSO4·5H2O (716 mg, 2.87 mmol) and sodium ascorbate (1.29 g, 6.51 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 98 (622 mg, 36%) as a pale yellow-green powdery solid. m.p. 102–104 °C. TLC Rf = 0.24 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2940w (C-H str), 2836w (C-H str), 1644m (C=O str), 1596m (C=C str), 1579m (C=C str), 1510s (C=C str), 1467m, 1420w, 1398w, 1260s, 1237s, 1196m, 1146s, 1138s, 1022s. 1H-NMR (500 MHz, CDCl3): δ 3.87 (3H, s, -OCH3), 3.94 (3H, s, -OCH3), 3.96 (3H, s, -OCH3), 4.38 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.74 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.32 (2H, s, -OCH2CN-), 6.84 (1H, d, J = 8.4 Hz, ArH), 6.90 (1H, d, J = 8.4 Hz, ArH), 7.16 (1H, d, J = 1.6 Hz, ArH), 7.24 (1H, dd, J = 8.4, 1.6 Hz, ArH), 7.39 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.39–7.44 (4H, m, ArH), 7.50 (1H, d, J = 8.4 Hz, ArH), 7.59 (1H, d, J = 1.6 Hz, ArH), 7.62 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.69 (1H, t, J = 8.4 Hz, ArH), 7.77 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.96 (1H, s, -CHN-), 7.97–8.00 (2H, m, ArH), 8.27 (1H, dd, J = 8.0, 1.6 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 56.0, 56.0, 65.1, 67.4, 110.2, 111.1, 111.5, 112.2, 118.1, 119.5, 122.4, 123.0, 124.1, 124.8, 125.3, 125.7, 127.9, 128.3, 128.7, 130.6, 130.7, 132.7, 133.5, 139.4, 143.9, 144.5, 149.2, 149.7, 151.3, 151.3, 155.3, 156.4, 175.0, 188.6. LCMS (ES+) m/z = 660.2 ([M + H]+, tR = 1.64 min). HRMS (ESI+) m/z = 660.2325 [M + H]+ found, C38H34O8N3+ required 660.2340.
(E)-3-((1-(2-(2-Methoxy-4-(3-(2,3,4-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (99). A mixture of alkyne flavone 30 (205 mg, 0.742 mmol), azide chalcone 68 (306 mg, 0.739 mmol), CuSO4·5H2O (204 mg, 0.818 mmol) and sodium ascorbate (365 mg, 1.84 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 99 (178 mg, 35%) as an off-white powdery solid. m.p. 120–122 °C. TLC Rf = 0.28 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2838w (C-H str), 1740w, 1645s (C=O str), 1581s (C=C str), 1563m (C=C str), 1516w (C=C str), 1494s, 1466s, 1414m, 1403m, 1264s, 1238m, 1195m, 1148m, 1096s, 1039m, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.90 (3H, s, -OCH3), 3.92 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 4.36 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.73 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.33 (2H, s, -OCH2CN-), 6.73 (1H, d, J = 8.8 Hz, ArH), 6.84 (1H, d, J = 8.0 Hz, ArH), 7.38–7.44 (5H, m, ArH), 7.50 (1H, d, J = 8.4 Hz, ArH), 7.55 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.59–7.62 (2H, m, ArH), 7.69 (1H, t, J = 8.4 Hz, ArH), 7.95 (1H, s, -CHN-), 7.96–7.99 (2H, m, ArH), 7.99 (1H, d, J = 15.6 Hz, -CH=CHCO-), 8.28 (1H, dd, J = 8.0, 1.2 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 55.9, 56.1, 60.9, 61.4, 65.1, 67.4, 107.6, 111.5, 112.2, 118.1, 120.9, 122.0, 122.4, 123.9, 124.1, 124.8, 125.3, 125.7, 128.3, 128.7, 130.6, 130.7, 132.8, 133.5, 139.4, 139.7, 142.5, 143.9, 149.6, 151.2, 153.7, 155.3, 155.7, 156.4, 175.0, 189.0. LCMS (ES+) m/z = 690.2 ([M + H]+, tR = 1.95 min). HRMS (ESI+) m/z = 690.2425 [M + H]+ found, C39H36O9N3+ required 690.2446.
(E)-3-((1-(2-(2-Methoxy-4-(3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (100). A mixture of alkyne flavone 30 (207 mg, 0.749 mmol), azide chalcone 69 (306 mg, 0.741 mmol), CuSO4·5H2O (203 mg, 0.814 mmol) and sodium ascorbate (369 mg, 1.86 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 100 (342 mg, 67%) as a white powdery solid. m.p. 172–174 °C. TLC Rf = 0.30 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2843w (C-H str), 1737w, 1643s (C=O str), 1599s (C=C str), 1575s (C=C str), 1515m (C=C str), 1465m, 1416m, 1322m, 1261m, 1231s, 1216m, 1192m, 1149s, 1125s, 1027s. 1H-NMR (500 MHz, CDCl3): δ 3.85 (3H, s, -OCH3), 3.88 (3H, s, -OCH3), 3.92 (6H, s, 2 × -OCH3), 4.35 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.72 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.35 (2H, s, -OCH2CN-), 6.16 (2H, s, ArH), 6.84 (1H, d, J = 8.8 Hz, ArH), 7.38–7.44 (4H, m, ArH), 7.50 (1H, d, J = 8.4 Hz, ArH), 7.60–7.62 (2H, m, ArH), 7.70 (1H, t, J = 8.8 Hz, ArH), 7.87 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.94 (1H, s -CHN-), 7.95–7.98 (2H, m, ArH), 8.25 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.29 (1H, dd, J = 8.4, 1.6 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 55.4, 55.8, 55.8, 65.0, 67.3, 90.5, 106.5, 111.7, 112.3, 118.1, 121.6, 122.3, 124.1, 124.7, 125.2, 125.7, 128.2, 128.7, 130.6, 130.7, 133.5, 133.5, 135.6, 139.3, 143.8, 149.4, 150.7, 155.2, 156.4, 161.6, 163.0, 175.0, 190.4. LCMS (ES+) m/z = 690.2 ([M + H]+, tR = 1.73 min). HRMS (ESI+) m/z = 690.2476 [M + H]+ found, C39H36O9N3+ required 690.2446.
(E)-6-((1-(2-(4-(3-(3,4-Dimethoxyphenyl)acryloyl)-2-methoxyphenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (101). A mixture of alkyne flavone 31 (179 mg, 0.648 mmol), azide chalcone 67 (246 mg, 0.641 mmol), CuSO4·5H2O (188 mg, 0.753 mmol) and sodium ascorbate (322 mg, 1.63 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 101 (366 mg, 87%) as a bright orange crystalline solid. m.p. 128–130 °C. TLC Rf = 0.34 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2940w (C-H str), 2831w (C-H str), 1637s (C=O str), 1618m, 1595s (C=C str), 1571s (C=C str), 1510s (C=C str), 1481m, 1454s, 1419m, 1360s, 1257s, 1235m, 1198m, 1140s, 1023s. 1H-NMR (500 MHz, CDCl3): δ 3.92 (3H, s, -OCH3), 3.93 (3H, s, -OCH3), 3.94 (3H, s, -OCH3), 4.47 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.86 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.29 (2H, s, -OCH2CN-), 6.79 (1H, s, -C=CH), 6.85 (1H, d, J = 8.8 Hz, ArH), 6.89 (1H, d, J = 8.4 Hz, ArH), 7.14 (1H, d, J = 1.6 Hz, ArH), 7.22 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.33 (1H, dd, J = 9.2, 3.2 Hz, ArH), 7.36 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.49–7.52 (4H, m, ArH), 7.59–7.61 (2H, m, ArH), 7.71 (1H, d, J = 3.2 Hz, ArH), 7.73 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.88–7.91 (2H, m, ArH), 8.04 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.9, 56.0, 62.3, 67.5, 106.3, 106.7, 110.1, 111.1, 111.5, 112.3, 119.4, 119.7, 122.5, 123.0, 123.9, 124.5, 124.6, 126.2, 127.8, 129.0, 131.5, 131.7, 132.7, 143.3, 144.5, 149.2, 149.7, 151.2, 151.2, 151.3, 155.5, 163.2, 178.1, 188.5. LCMS (ES+) m/z = 660.2 ([M + H]+, tR = 1.81 min). HRMS (ESI+) m/z = 682.2153 [M + Na]+ found, C38H33O8N3Na+ required 682.2160.
(E)-6-((1-(2-(2-Methoxy-4-(3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (102). A mixture of alkyne flavone 31 (211 mg, 0.763 mmol), azide chalcone 69 (317 mg, 0.767 mmol), CuSO4·5H2O (211 mg, 0.843 mmol) and sodium ascorbate (382 mg, 1.93 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 102 (397 mg, 75%) as a bright yellow-orange flaky solid. m.p. 128–130 °C. TLC Rf = 0.25 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2838w (C-H str), 1739w, 1640s (C=O str), 1598s (C=C str), 1569s (C=C str), 1517m (C=C str), 1455s, 1363m, 1320m, 1279m, 1261m, 1201s, 1154s, 1122s, 1035s, 1025s. 1H-NMR (500 MHz, CDCl3): δ 3.84 (3H, s, -OCH3), 3.89 (6H, s, 2 × -OCH3), 3.92 (3H, s, -OCH3), 4.45 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.85 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.28 (2H, s, -OCH2CN-), 6.12 (2H, s, ArH), 6.79 (1H, s, -C=CH), 6.84 (1H, d, J = 8.4 Hz, ArH), 7.33 (1H, dd, J = 9.2, 3.2 Hz, ArH), 7.48–7.51 (4H, m, ArH), 7.57 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.61 (1H, d, J = 1.6 Hz, ArH), 7.71 (1H, d, J = 2.8 Hz, ArH), 7.84 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.88–7.91 (2H, m, ArH), 8.06 (1H, s, -CHN-), 8.21 (1H, d, J = 15.6 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.3, 55.7, 55.9, 62.3, 67.5, 90.5, 106.2, 106.5, 106.7, 111.6, 112.4, 119.6, 121.4, 122.3, 123.9, 124.4, 124.6, 126.2, 128.9, 131.5, 131.7, 133.6, 135.5, 143.3, 149.4, 150.6, 151.2, 155.6, 161.6, 163.0, 163.2, 178.1, 190.3. LCMS (ES+) m/z = 690.3 ([M + H]+, tR = 1.88 min). HRMS (ESI+) m/z = 690.2426 [M + H]+ found, C39H36O9N3+ required 690.2446.
(E)-6-((1-(2-(2-Methoxy-4-(3-(2,3,4-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-2-phenyl-4H-chromen-4-one (103). A mixture of alkyne flavone 31 (201 mg, 0.729 mmol), azide chalcone 68 (304 mg, 0.735 mmol), CuSO4·5H2O (208 mg, 0.831 mmol) and sodium ascorbate (383 mg, 1.93 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 103 (382 mg, 76%) as a pale yellow flaky solid. m.p. 138–140 °C. TLC Rf = 0.32 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2939w (C-H str), 2839w (C-H str), 1740w, 1640s (C=O str), 1619m, 1593m (C=C str), 1573s (C=C str), 1516m (C=C str), 1495m, 1482m, 1455s, 1415m, 1360s, 1259s, 1200m, 1157m, 1095s, 1026s. 1H-NMR (500 MHz, CDCl3): δ 3.89 (3H, s, -OCH3), 3.90 (3H, s, -OCH3), 3.93 (3H, s, -OCH3), 3.94 (3H, s, -OCH3), 4.47 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.86 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.29 (2H, s, -OCH2CN-), 6.72 (1H, d, J = 8.8 Hz, ArH), 6.80 (1H, s, -C=CH), 6.86 (1H, d, J = 8.0 Hz, ArH), 7.34 (1H, dd, J = 9.2, 3.2 Hz, ArH), 7.37 (1H, d, J = 8.8 Hz, ArH), 7.49–7.52 (4H, m, ArH), 7.53 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.58–7.61 (2H, m, ArH), 7.71 (1H, d, J = 3.2 Hz, ArH), 7.89–7.92 (2H, m, ArH), 7.96 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.05 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.0, 56.0, 60.9, 61.3, 62.3, 67.5, 106.3, 106.8, 107.5, 111.5, 112.3, 119.7, 120.8, 122.0, 122.4, 123.8, 123.9, 124.5, 124.6, 126.2, 129.0, 131.5, 131.7, 132.8, 139.6, 142.4, 143.3, 149.6, 151.1, 151.2, 153.7, 155.6, 155.7, 163.2, 178.1, 188.9. LCMS (ES+) m/z = 690.2 ([M + H]+, tR = 1.94 min). HRMS (ESI+) m/z = 690.2452 [M + H]+ found, C39H36N3O9+ required 690.2452.
(E)-7-((1-(2-(2-Methoxy-4-(3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-2-phenyl-4H-chromen-4-one (104). A mixture of alkyne flavone 32 (202 mg, 0.730 mmol), azide chalcone 69 (304 mg, 0.735 mmol), CuSO4·5H2O (218 mg, 0.872 mmol) and sodium ascorbate (386 mg, 1.95 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 104 (324 mg, 64%) as a pale yellow-white powdery solid. m.p. 168–170 °C. TLC Rf = 0.25 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2841w (C-H str), 1736w, 1634s (C=O str), 1599s (C=C str), 1566s (C=C str), 1512m (C=C str), 1451m, 1441m, 1336s, 1302w, 1279m, 1261m, 1204m, 1175m, 1160w, 1122s, 1092w, 1053w, 1037m, 1016w. 1H-NMR (500 MHz, CDCl3): δ 3.87 (3H, s, -OCH3), 3.91 (6H, s, 2 × -OCH3), 3.93 (3H, s, -OCH3), 4.48 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.87 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.35 (2H, s, -OCH2CN-), 6.14 (2H, s, ArH), 6.77 (1H, s, -C=CH), 6.86 (1H, d, J = 8.0 Hz, ArH), 7.06 (1H, dd, J = 8.8, 2.0 Hz, ArH), 7.14 (1H, d, J = 2.0 Hz, ArH), 7.49–7.53 (3H, m, ArH), 7.59 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.63 (1H, d, J = 1.6 Hz, ArH), 7.84 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.90–7.92 (2H, m, ArH), 8.07 (1H, s, -CHN-), 8.15 (1H, d, J = 8.8 Hz, ArH), 8.23 (1H, d, J = 16.0 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.3, 55.7, 55.8, 62.3, 67.4, 90.4, 101.4, 106.4, 107.3, 111.6, 112.4, 114.8, 118.0, 121.3, 122.3, 124.7, 126.1, 127.0, 128.9, 131.4, 131.6, 133.6, 135.6, 143.0, 149.4, 150.5, 157.8, 161.6, 162.6, 163.0, 163.1, 177.7, 190.2. LCMS (ES+) m/z = 690.2 ([M + H]+, tR = 1.70 min). HRMS (ESI+) m/z = 690.2471 [M + H]+ found, C39H36O9N3+ required 690.2446.
(E)-2-(4-(2-(4-((2-(3-(Ferrocenyl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-hydroxy-7-methoxy-4H-chromen-4-one (105). A mixture of alkyne chalcone 26 (310 mg, 0.838 mmol), azide flavonol 80 (296 mg, 0.838 mmol), CuSO4·5H2O (273 mg, 1.09 mmol) and sodium ascorbate (496 mg, 2.50 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 105 (238 mg, 40%) as a dark red powdery solid. m.p. 118–120 °C. TLC Rf = 0.44 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3340w (O-H str), 3088w (C-H str), 2929w (C-H str), 1735m (C=O str), 1597s (C=C str), 1541m (C=C str), 1506m (C=C str), 1484m, 1449m, 1403m, 1235s, 1206s, 1171s, 1117m, 1106m, 1044w, 1025s. 1H-NMR (500 MHz, CDCl3): δ 3.96 (3H, s, -OCH3), 4.12 (5H, s, -C5H5), 4.35 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 4.43 (2H, t, J = 1.5 Hz, -C5H4), 4.48 (2H, t, J = 1.5 Hz, -C5H4), 4.67 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.33 (2H, br s, -OCH2CN-), 6.95–6.97 (4H, m, ArH and OH), 6.96 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.01 (1H, dd, J = 9.0, 2.0 Hz, ArH), 7.09 (1H, t, J = 7.5 Hz, ArH), 7.14 (1H, d, J = 8.5 Hz, ArH), 7.46 (1H, dd, J = 7.5, 1.0 Hz, ArH), 7.50 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.60 (1H, dd, J = 7.5, 1.5 Hz, ArH), 7.77 (1H, br s, -CHN-), 8.14 (1H, d, J = 9.0 Hz, ArH), 8.19 (2H, d, J = 8.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.6, 55.8, 63.0, 66.1, 68.9, 69.8, 71.3, 79.0, 99.9, 113.1, 114.5, 114.6, 114.7, 121.4, 124.0, 124.7, 126.7, 129.3, 130.1, 130.2, 132.4, 137.4, 144.1, 145.5, 156.3, 157.2, 158.8, 164.2, 172.6, 192.3. LCMS (ES+) m/z = 724.2 ([M + H]+, tR = 2.01 min). HRMS (ESI+) m/z = 724.1711 [M + H]+ found, C40H34N3O7Fe+ required 724.1746.
(E)-2-(4-(2-(4-((4-(3-(Ferrocenyl)acryloyl)-2-methoxyphenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-hydroxy-4H-chromen-4-one (106). A mixture of alkyne chalcone 25 (308 mg, 0.769 mmol), azide flavonol 79 (252 mg, 0.779 mmol), CuSO4·5H2O (209 mg, 0.835 mmol) and sodium ascorbate (395 mg, 1.99 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 106 (212 mg, 38%) as a dark-red powdery solid. m.p. 158–160 °C. TLC Rf = 0.26 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3429w (O-H str), 2943w (C-H str), 2873w (C-H str), 1735m (C=O str), 1645w, 1596s (C=C str), 1568s (C=C str), 1509s (C=C str), 1469m, 1418m, 1409m, 1348w, 1292w, 1254s, 1200w, 1180m, 1151m, 1108m, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.97 (3H, s, -OCH3), 4.17 (5H, s, -C5H5), 4.46–4.48 (4H, m, -C5H4 and -OCH2CH2N-), 4.57 (2H, t, J = 2.0 Hz, -C5H4), 4.83 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.43 (2H, s, -OCH2CN-), 6.94 (1H, br s, OH), 6.98 (2H, d, J = 9.0 Hz, ArH), 7.12 (1H, d, J = 15.0 Hz, -CH=CHCO-), 7.15 (1H, d, J = 8.5 Hz, ArH), 7.43 (1H, t, J = 8.0 Hz, ArH), 7.57 (1H, dd, J = 8.0, 1.5 Hz, ArH), 7.59 (1H, d, J = 8.5 Hz, ArH), 7.63 (1H, d, J = 2.0 Hz, ArH), 7.71 (1H, t, J = 8.0 Hz, ArH), 7.73 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.90 (1H, br s, -CHN-), 8.22 (2H, d, J = 9.0 Hz, ArH), 8.25 (1H, dd, J = 8.0, 1.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.1, 62.9, 66.3, 68.9, 69.7, 71.2, 79.3, 111.2, 112.3, 114.5, 118.2, 118.5, 120.7, 122.4, 124.3, 124.5, 124.6, 125.4, 129.6, 132.3, 133.5, 137.8, 143.8, 144.7, 146.0, 149.5, 151.3, 155.2, 159.0, 173.2, 187.9. LCMS (ES+) m/z = 724.2 ([M + H]+, tR = 2.04 min). HRMS (ESI+) m/z = 724.1723 [M + H]+ found, C40H34N3O7Fe+ required 724.1746.
(E)-2-(3-(2-(4-((4-Bromo-2-(3-(1-methyl-1H-indol-3-yl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-ethoxy)-4-methoxyphenyl)-3-hydroxy-4H-chromen-4-one (107). A mixture of alkyne chalcone 21 (339 mg, 0.859 mmol), azide flavonol 82 (301 mg, 0.853 mmol), CuSO4·5H2O (542 mg, 2.17 mmol) and sodium ascorbate (202 mg, 1.02 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 107 (258 mg, 40%) as a brown powdery solid. m.p. 158–160 °C. TLC Rf = 0.44 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3351w (O-H str), 2920m (C-H str), 2852w (C-H str), 1640m (C=O str), 1589s (C=C str), 1559m (C=C str), 1514m (C=C str), 1492m, 1471m, 1397m, 1374m, 1333w, 1269s, 1204m, 1180m, 1130s, 1074w, 1047m, 1020m. 1H-NMR (500 MHz, CDCl3): δ 3.64 (3H, s, -NCH3), 3.83 (3H, s, -OCH3), 4.02 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 4.49 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.36 (2H, br s, -OCH2CN-), 6.96 (1H, d, J = 9.0 Hz, ArH), 7.02 (1H, br s, OH), 7.07 (1H, d, J = 8.5 Hz, ArH), 7.10–7.13 (1H, m, ArH), 7.18 (1H, s, ArH), 7.23 (2H, d, J = 3.5 Hz, ArH), 7.38 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.45 (1H, t, J = 7.5 Hz, ArH), 7.50 (1H, d, J = 1.5 Hz, ArH), 7.53 (1H, d, J = 7.5 Hz, ArH), 7.56 (1H, dd, J = 8.5, 2.5 Hz, ArH), 7.60 (1H, d, J = 8.5 Hz, ArH), 7.73 (1H, t, J = 7.0 Hz, ArH), 7.77 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.83 (1H, d, J = 2.5 Hz, ArH), 7.90 (1H, dd, J = 8.5, 1.5 Hz, ArH), 8.06 (1H, br s, -CHN-), 8.27 (1H, d, J = 8.0 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 33.0, 49.5, 55.9, 63.5, 67.1, 109.9, 111.4, 112.6, 113.2, 114.0, 114.8, 118.1, 120.5, 120.6, 121.4, 121.6, 122.6, 123.0, 123.3, 123.7, 124.6, 124.8, 125.4, 125.7, 131.9, 133.3, 133.6, 134.8, 134.9, 137.7, 137.8, 138.0, 144.5, 146.9, 151.3, 155.1, 155.8, 173.0, 190.5. LCMS (ES+) m/z = 749.2 ([M + H]+, tR = 1.99 min). HRMS (ESI+) m/z = 769.1232 [M + Na]+ found, C39H31O7N4BrNa+ required 769.1268.
(E)-2-(4-(2-(4-((4-Bromo-2-(3-(furan-2-yl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)-3-methoxyphenyl)-3-hydroxy-4H-chromen-4-one (108). A mixture of alkyne chalcone 24 (289 mg, 0.872 mmol), azide flavonol 81 (304 mg, 0.860 mmol), CuSO4·5H2O (234 mg, 0.936 mmol) and sodium ascorbate (476 mg, 2.40 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 108 (245 mg, 42%) as a dark yellow-brown powdery solid. m.p. 118–120 °C. TLC Rf = 0.41 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3275w (O-H str), 3110w (C-H str), 2926w (C-H str), 1650m (C=O str), 1598s (C=C str), 1549m (C=C str), 1515s (C=C str), 1481s, 1422w, 1398m, 1267s, 1232m, 1207s, 1177m, 1145s, 1111m, 1041m, 1017m, 1008m. 1H-NMR (500 MHz, CDCl3): δ 3.90 (3H, s, -OCH3), 4.43 (2H, t, J = 4.0 Hz, -OCH2CH2N-), 4.75 (2H, t, J = 4.0 Hz, -OCH2CH2N-), 5.33 (2H, br s, -OCH2CN-), 6.47 (1H, d, J = 1.5 Hz, ArH), 6.64 (1H, d, J = 3.0 Hz, ArH), 6.89 (1H, d, J = 8.5 Hz, ArH), 7.01 (1H, s, ArH), 7.06–7.08 (1H, m, ArH), 7.25 (1H, d, J = 16.0 Hz, -CH=CHCO-, overlain by CDCl3), 7.36 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.44 (1H, t, J = 7.5 Hz, ArH), 7.52 (1H, s, ArH), 7.54 (1H, d, J = 6.5 Hz, ArH), 7.61 (1H, d, J = 8.5 Hz, ArH), 7.72 (1H, d, J = 7.0 Hz, ArH), 7.74 (1H, d, J = 2.0 Hz, ArH), 7.82 (1H, d, J = 8.5 Hz, ArH), 7.84 (1H, s, -CHN-), 8.09 (1H, br s, OH), 8.26 (1H, d, J = 7.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.9, 55.8, 63.2, 67.5, 111.4, 112.7, 113.6, 113.9, 115.1, 116.1, 118.2, 120.6, 121.1, 124.0, 124.5, 125.2, 125.4, 129.6, 131.1, 133.0, 133.5, 135.4, 137.9, 144.6, 145.0, 148.7, 149.4, 151.4, 155.2, 155.7, 173.1, 190.2. LCMS (ES+) m/z = 686.2 ([M + H]+, tR = 2.00 min). HRMS (ESI+) m/z = 684.0955 [M + H]+ found, C34H27O8N3Br+ required 684.0976.
6-(2-(4-(((4-Oxo-2-phenyl-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)-2-phenyl-4H-chromen-4-one (109). A mixture of alkyne flavone 30 (277 mg, 1.00 mmol), azide flavone 85 (307 mg, 0.998 mmol), CuSO4·5H2O (303 mg, 1.21 mmol) and sodium ascorbate (492 mg, 2.48 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 109 (534 mg, 92%) as a white powdery solid. m.p. 234–236 °C. TLC Rf = 0.36 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3145w (C-H str), 2940w (C-H str), 1736w, 1643s (C=O str), 1627s, 1600m, 1570m (C=C str), 1561m (C=C str), 1482m, 1470m, 1455s, 1398m, 1361s, 1293m, 1197s, 1187m, 1148m, 1087m, 1047m, 1026w. 1H-NMR (500 MHz, CDCl3): δ 4.41 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.73 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.34 (2H, s, -OCH2CN-), 6.83 (1H, s, -C=CH), 7.25 (1H, dd, J = 9.2, 3.2 Hz, ArH, overlain by CDCl3), 7.41–7.46 (4H, m, ArH), 7.51–7.55 (5H, m, ArH), 7.58 (1H, d, J = 3.2 Hz, ArH), 7.70 (1H, t, J = 7.2 Hz, ArH), 7.81 (1H, s, -CHN-), 7.92–7.94 (2H, m, ArH), 8.01–8.04 (2H, m, ArH), 8.29 (1H, d, J = 7.2 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 65.1, 66.7, 105.9, 106.9, 118.0, 119.9, 123.8, 124.1, 124.5, 124.8, 125.7, 126.2, 128.3, 128.7, 129.0, 130.7, 131.6, 131.7, 133.5, 139.5, 144.0, 151.4, 155.1, 155.3, 156.4, 163.3, 175.1, 178.0. LCMS (ES+) m/z = 584.1 ([M + H]+, tR = 1.91 min). HRMS (ESI+) m/z = 606.1610 [M + Na]+ found, C35H25O6N3Na+ required 606.1636.
3-Hydroxy-2-(4-(2-(4-(((4-oxo-2-phenyl-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl) -4H-chromen-4-one (110). A mixture of alkyne flavone 30 (272 mg, 0.984 mmol), azide flavonol 79 (311 mg, 0.961 mmol), CuSO4·5H2O (274 mg, 1.10 mmol) and sodium ascorbate (557 mg, 2.81 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 110 (272 mg, 47%) as an off-white powdery solid. m.p. 148–150 °C. TLC Rf = 0.34 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3254w(br) (O-H str), 2964w (C-H str), 1602s (C=O str), 1564m (C=C str), 1549m (C=C str), 1509s (C=C str), 1481m, 1469s, 1427m, 1403s, 1282m, 1250s, 1198m, 1183s, 1150w, 1116m, 1109m, 1041m. 1H-NMR (500 MHz, CDCl3): δ 4.39 (2H, t, J = 5.5 Hz, -OCH2CH2N-), 4.73 (2H, t, J = 5.5 Hz, -OCH2CH2N-), 5.33 (2H, s, -OCH2CN-), 6.99 (3H, d, J = 9.0 Hz, ArH and OH), 7.41–7.46 (5H, m, ArH), 7.53 (1H, dd, J = 8.0, 0.5 Hz, ArH), 7.59 (1H, d, J = 8.0 Hz, ArH), 7.68–7.72 (2H, m, ArH), 7.85 (1H, s, -CHN-), 8.01–8.03 (2H, m, ArH), 8.23 (2H, d, J = 9.0 Hz, ArH), 8.25 (1H, dd, J = 8.0, 1.5 Hz, ArH), 8.29 (1H, dd, J = 8.0, 1.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.5, 65.1, 66.2, 114.5, 118.1, 118.2, 120.7, 124.1, 124.5, 124.5, 124.8, 125.0, 125.4, 125.7, 128.4, 128.7, 129.6, 130.7, 133.5, 133.6, 137.8, 139.5, 144.1, 144.8, 155.3, 155.3, 156.4, 159.1, 173.2, 175.1. LCMS (ES+) m/z = 600.0 ([M + H]+, tR = 1.75 min). HRMS (ESI+) m/z = 600.1755 [M + H]+ found, C35H26O7N3+ required 600.1765.
3-Hydroxy-2-(4-((1-(2-(4-(3-hydroxy-4-oxo-4H-chromen-2-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)phenyl)-4H-chromen-4-one (111). A mixture of alkyne flavonol 36 (273 mg, 0.933 mmol), azide flavonol 79 (310 mg, 0.958 mmol), CuSO4·5H2O (296 mg, 1.19 mmol) and sodium ascorbate (461 mg, 2.33 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 111 (82.7 mg, 14%) as a dark brown powdery solid. m.p. 218–220 °C. TLC Rf = 0.41 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3284w (O-H str), 3087w (C-H str), 2924w (C-H str), 1600s (C=O str), 1563m (C=C str), 1543m (C=C str), 1508s (C=C str), 1491s, 1424s, 1409s, 1248s, 1209m, 1180s, 1108s, 1043m, 1014w. 1H-NMR (500 MHz, DMSO-d6): δ 4.53 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 4.85 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.29 (2H, s, -OCH2CN-), 7.11 (2H, d, J = 8.5 Hz, ArH), 7.25 (2H, d, J = 8.5 Hz, ArH), 7.43–7.47 (2H, m, ArH), 7.71–7.80 (4H, m, ArH), 8.09 (2H, t, J = 6.5 Hz, ArH), 8.17 (2H, d, J = 9.0 Hz, ArH), 8.21 (2H, d, J = 9.0 Hz, ArH), 8.39 (1H, s, -CHN-), 9.47 (1H, s, OH), 9.48 (1H, s, OH). 13C-NMR (500 MHz, DMSO-d6): δ 49.0, 61.1, 66.3, 114.6, 114.8, 115.4, 118.3, 121.3, 123.9, 124.1, 124.5, 124.7, 125.4, 129.4, 129.6, 133.4, 133.5, 138.2, 142.4, 145.3, 145.4, 154.4, 158.9, 159.1, 172.6. LCMS (ES+) m/z = 616.1 ([M + H]+, tR = 1.68 min). HRMS (ESI+) m/z = 616.1722 [M + H]+ found, C35H26N3O8+ required 616.1720.
3-(4-Methoxyphenyl)-7-((1-(2-((4-oxo-2-phenyl-4H-chromen-6-yl)oxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-4H-chromen-4-one (112). A mixture of alkyne isoflavone 46 (280 mg, 0.914 mmol), azide flavone 85 (282 mg, 0.919 mmol), CuSO4·5H2O (279 mg, 1.12 mmol) and sodium ascorbate (503 mg, 2.54 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 112 (531 mg, 95%) as a white powdery solid. m.p. 234–236 °C. TLC Rf = (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3082w (C-H str), 2941w (C-H str), 1641s, 1625s (C=O str), 1608s, 1567s (C=C str), 1515s (C=C str), 1497w, 1483w, 1456s, 1443s, 1359s, 1292s, 1252s, 1204m, 1192m, 1185s, 1137w, 1099m, 1084m, 1047s, 1032s. 1H-NMR (500 MHz, CDCl3): δ 3.83 (3H, s, -OCH3), 4.50 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.86 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.36 (2H, s, -OCH2CN-), 6.82 (1H, s, -C=CH), 6.95 (2H, d, J = 8.4 Hz, ArH), 7.01 (1H, d, J = 2.0 Hz, ArH), 7.07 (1H, dd, J = 8.8, 2.0 Hz, ArH), 7.18 (1H, dd, J = 8.8, 3.2 Hz, ArH), 7.48–7.56 (6H, m, ArH), 7.59 (1H, d, J = 3.2 Hz, ArH), 7.86 (1H, s, -CHN-), 7.90–7.92 (3H, m, -C=CH and ArH), 8.22 (1H, d, J = 8.4 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.3, 62.4, 66.8, 101.3, 106.2, 106.9, 113.9, 115.0, 118.8, 119.9, 123.4, 124.0, 124.1, 124.6, 124.9, 126.3, 127.9, 129.1, 130.1, 131.7, 143.3, 151.5, 152.1, 155.0, 157.7, 159.6, 162.3, 163.5, 175.8, 178.0. LCMS (ES+) m/z = 614.2 ([M + H]+, tR = 1.94 min). HRMS (ESI+) m/z = 614.1942 [M + H]+ found, C36H28N3O7+ required 614.1927.
(E)-7-Methoxy-3-(4-((1-(2-(2-methoxy-4-(3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-4H-chromen-4-one (113). A mixture of alkyne isoflavone 45 (236 mg, 0.770 mmol), azide chalcone 69 (308 mg, 0.744 mmol), CuSO4·5H2O (210 mg, 0.843 mmol) and sodium ascorbate (363 mg, 1.83 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH/PE to afford triazole hybrid 113 (167 mg, 31%) as a pale yellow-orange powdery solid. m.p. 118–120 °C. TLC Rf = 0.33 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2942w (C-H str), 2840w (C-H str), 1637m (C=O str), 1595s (C=C str), 1559s (C=C str), 1511s (C=C str), 1438m, 1418m, 1336m, 1300m, 1244s, 1201m, 1153s, 1177s, 1118s, 1037s, 1024s. 1H-NMR (500 MHz, CDCl3): δ 3.86 (3H, s, -OCH3), 3.90 (6H, s, 2 × -OCH3), 3.90 (3H, s, -OCH3), 3.93 (3H, s, -OCH3), 4.46 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.26 (2H, s, -OCH2CN-), 6.13 (2H, s, ArH), 6.83 (1H, d, J = 0.4 Hz, ArH), 6.84 (1H, d, J = 6.0 Hz, ArH), 6.97 (1H, dd, J = 8.8, 2.4 Hz, ArH), 7.04 (2H, d, J = 8.8 Hz, ArH), 7.48 (2H, d, J = 8.8 Hz, ArH), 7.58 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.62 (1H, d, J = 2.0 Hz, ArH), 7.84 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.88 (1H, s, -CHN-), 8.02 (1H, s, -C=CH), 8.19 (1H, d, J = 8.8 Hz, ArH), 8.21 (1H, d, J = 15.6 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.0, 55.4, 55.8, 55.9, 62.1, 67.6, 90.5, 100.0, 106.6, 111.7, 112.5, 114.5, 114.8, 118.4, 121.6, 122.3, 122.9, 124.4, 127.7, 130.1, 133.7, 135.6, 144.3, 149.5, 150.7, 152.1, 157.9, 158.2, 158.3, 161.6, 163.0, 163.9, 175.7, 190.3. LCMS (ES+) m/z = 720.3 ([M + H]+, tR = 1.69 min). HRMS (ESI+) m/z = 720.2529 [M + H]+ found, C40H38O10N3+ required 720.2552.
(E)-7-((1-(2-(2-Methoxy-4-(3-(2,3,4-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (114). A mixture of alkyne isoflavone 46 (226 mg, 0.738 mmol), azide chalcone 68 (301 mg, 0.728 mmol), CuSO4·5H2O (204 mg, 0.818 mmol) and sodium ascorbate (393 mg, 1.98 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 114 (331 mg, 63%) as a pale yellow-green powdery solid. m.p. 98–100 °C. TLC Rf = 0.39 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2937w (C-H str), 2838w (C-H str), 1623s (C=O str), 1594s (C=C str), 1576s (C=C str), 1512s (C=C str), 1494s, 1463m, 1442m, 1416m, 1247s, 1196m, 1178m, 1094s, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.83 (3H, s, -OCH3), 3.89 (3H, s, -OCH3), 3.91 (3H, s, -OCH3), 3.93 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 4.47 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.86 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.32 (2H, s, -OCH2CN-), 6.72 (1H, d, J = 8.4 Hz, ArH), 6.85 (1H, d, J = 8.4 Hz, ArH), 6.95 (2H, d, J = 8.8 Hz, ArH), 7.01 (1H, d, J = 2.0 Hz, ArH), 7.04 (1H, dd, J = 8.8, 2.0 Hz, ArH), 7.38 (1H, d, J = 8.8 Hz, ArH), 7.49 (2H, d, J = 8.8 Hz, ArH), 7.53 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.60 (1H, d, J = 8.4, 1.6 Hz, ArH), 7.62 (1H, d, J = 0.8 Hz, ArH), 7.90 (1H, s, -C=CH), 7.98 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.06 (1H, s, -CHN-), 8.20 (1H, d, J = 9.2 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.3, 56.0, 56.0, 60.9, 61.4, 62.4, 67.5, 101.2, 107.6, 111.5, 112.4, 113.9, 114.8, 118.7, 120.7, 122.0, 122.4, 123.9, 124.1, 124.7, 124.8, 127.9, 130.1, 133.0, 139.7, 142.4, 143.1, 149.6, 151.0, 152.1, 153.7, 155.7, 157.7, 159.5, 162.4, 175.7, 188.9. LCMS (ES+) m/z = 720.3 ([M + H]+, tR = 1.84 min). HRMS (ESI+) m/z = 720.2533 [M + H]+ found, C40H38O10N3+ required 720.2552.
3-Hydroxy-2-(4-(2-(4-(((3-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)-ethoxy)phenyl)-4H-chromen-4-one (115). A mixture of alkyne isoflavone 46 (183 mg, 0.597 mmol), azide flavonol 79 (189 mg, 0.585 mmol), CuSO4·5H2O (271 mg, 1.09 mmol) and sodium ascorbate (493 mg, 2.49 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 115 (42.9 mg, 12%) as a yellow-green powdery solid. m.p. 252–254 °C. TLC Rf = 0.30 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3371w(br) (O-H str), 3064w (C-H str), 2928w (C-H str), 1640m, 1626m (C=O str), 1601s, 1563m (C=C str), 1512m (C=C str), 1484w, 1444m, 1409m, 1290w, 1247s, 1201m, 1183m, 1121m, 1049m, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.83 (3H, s, -OCH3), 4.48 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.86 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.36 (2H, s, -OCH2CN-), 6.92 (2H, d, J = 9.0 Hz, ArH), 6.97 (3H, d, J = 9.0 Hz, ArH and OH), 7.00 (1H, d, J = 1.5 Hz, ArH), 7.07 (1H, dd, J = 8.5, 2.0 Hz, ArH), 7.42 (1H, t, J = 7.5 Hz, ArH), 7.47 (2H, d, J = 8.5 Hz, ArH), 7.57 (1H, d, J = 8.0 Hz, ArH), 7.70 (1H, t, J = 8.5 Hz, ArH), 7.89 (2H, s, -C=CH and -CHN-), 8.21–8.25 (4H, m, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.3, 62.4, 66.3, 101.3, 113.9, 114.5, 115.0, 118.2, 118.8, 120.6, 124.1, 124.5, 124.7, 124.9, 125.4, 127.9, 129.6, 130.0, 133.5, 137.8, 144.6, 152.1, 155.3, 157.7, 159.0, 159.5, 162.3, 173.2, 175.8. LCMS (ES+) m/z = 630.2 ([M + H]+, tR = 1.77 min). HRMS (ESI+) m/z = 630.1861 [M + H]+ found, C36H28O8N3+ required 630.1871.
2-((Z)-4-(2-(4-((2-Methoxy-4-((E)-3-(4-methoxyphenyl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)-ethoxy)benzylidene)benzofuran-3(2H)-one (116). A mixture of alkyne chalcone 20 (192 mg, 0.595 mmol), azide aurone 88 (187 mg, 0.609 mmol), CuSO4·5H2O (289 mg, 1.16 mmol) and sodium ascorbate (548 mg, 2.77 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 116 (148 mg, 40%) as a bright yellow powdery solid. m.p. 128–130 °C. TLC Rf = 0.33 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2941w (C-H str), 2835w (C-H str), 1695m (C=O str), 1647m, 1590s (C=C str), 1571s (C=C str), 1509s (C=C str), 1461m, 1422m, 1296m, 1250s, 1174s, 1147s, 1128s, 1110s, 1098m, 1025s. 1H-NMR (500 MHz, CDCl3): δ 3.85 (3H, s, -OCH3), 3.97 (3H, s, -OCH3), 4.43 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.80 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.42 (2H, s, -OCH2CN-), 6.83 (1H, s, -C=CH), 6.89 (2H, d, J = 9.0 Hz, ArH), 6.91 (2H, d, J = 9.0 Hz, ArH), 7.12 (1H, d, J = 8.0 Hz, ArH), 7.22 (1H, t, J = 8.0 Hz, ArH), 7.33 (1H, d, J = 8.0 Hz, ArH), 7.39 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.56 (2H, d, J = 8.5 Hz, ArH), 7.60 (1H, dd, J = 8.5, 2.0 Hz, ArH), 7.64–7.67 (2H, m, ArH), 7.77 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.79–7.81 (1H, m, ArH), 7.86 (2H, d, J = 9.0 Hz, ArH), 7.87 (1H, br s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.4, 56.1, 62.9, 66.3, 111.1, 112.3, 112.6, 112.9, 114.3, 114.9, 119.1, 121.8, 122.6, 123.3, 124.3, 124.6, 126.1, 127.7, 130.1, 132.2, 133.1, 133.4, 136.6, 144.0, 146.1, 149.6, 151.4, 158.9, 161.5, 165.9, 184.5, 188.6. LCMS (ES+) m/z = 630.0 ([M + H]+, tR = 1.72 min). HRMS (ESI+) m/z = 630.2217 [M + H]+ found, C37H32O7N3+ required 630.2235.
2-((Z)-4-(2-(4-((2-((E)-3-(Ferrocenyl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)benzylidene)benzofuran-3(2H)-one (117). A mixture of alkyne chalcone 26 (286 mg, 0.772 mmol), azide aurone 88 (248 mg, 0.806 mmol), CuSO4·5H2O (236 mg, 0.943 mmol) and sodium ascorbate (426 mg, 2.15 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 117 (409 mg, 78%) as a dark red-purple microcrystalline solid. m.p. 108–110 °C. TLC Rf = 0.48 (3% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3090w (C-H str), 1699m (C=O str), 1646m, 1594s (C=C str), 1569m (C=C str), 1509s (C=C str), 1476w, 1459m, 1347w, 1297m, 1247m, 1208m, 1178s, 1127s, 1109s, 1097s, 1044m, 1025m, 1001w. 1H-NMR (500 MHz, CDCl3): δ 4.11 (5H, s, -C5H5), 4.32 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.42 (2H, t, J = 2.0 Hz, -C5H4), 4.47 (2H, t, J = 2.0 Hz, -C5H4), 4.65 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.32 (2H, s, -OCH2CN-), 6.85 (1H, s, -C=CH), 6.88 (2H, d, J = 8.8 Hz, ArH), 6.95 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.08 (1H, t, J = 7.6 Hz, ArH), 7.13 (1H, d, J = 8.4 Hz, ArH), 7.22 (1H, t, J = 7.6 Hz, ArH), 7.34 (1H, d, J = 8.4 Hz, ArH), 7.44–7.46 (1H, m, ArH), 7.49 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.60 (1H, dd, J = 7.6, 1.6 Hz, ArH), 7.65 (1H, t, J = 8.4 Hz, ArH), 7.74 (1H, s, -CHN-), 7.81 (1H, dd, J = 7.6, 0.8 Hz, ArH), 7.86 (2H, d, J = 8.8 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.5, 62.9, 66.1, 68.9, 69.8, 71.2, 79.0, 112.8, 112.9, 113.1, 115.0, 121.4, 121.8, 123.3, 123.9, 124.6, 124.7, 125.9, 130.1, 130.2, 132.4, 133.4, 136.6, 144.1, 145.4, 146.0, 156.3, 159.0, 165.8, 184.5, 192.3. LCMS (ES+) m/z = 678.2 ([M + H]+, tR = 2.11 min). HRMS (ESI+) m/z = 678.1677 [M + H]+ found, C39H32N3O5Fe+ required 678.1691.
6-Methoxy-2-((Z)-4-(2-(4-((2-methoxy-5-((E)-3-(1-methyl-1H-pyrrol-2-yl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)benzylidene)benzofuran-3(2H)-one (118). A mixture of alkyne chalcone 22 (265 mg, 0.898 mmol), azide aurone 89 (304 mg, 0.900 mmol), CuSO4·5H2O (264 mg, 1.06 mmol) and sodium ascorbate (474 mg, 2.39 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 118 (270 mg, 48%) as a bright yellow powdery solid. m.p. 138–140 °C. TLC Rf = 0.46 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2934w (C-H str), 2884w (C-H str), 1700m (C=O str), 1656m, 1638m, 1596s (C=C str), 1562s (C=C str), 1510m (C=C str), 1483m, 1412m, 1330m, 1267s, 1198m, 1152s, 1132s, 1099s, 1056m, 1042s, 1022s. 1H-NMR (500 MHz, CDCl3): δ 3.74 (3H, s, -NCH3), 3.94 (3H, s, -OCH3), 3.96 (3H, s, -OCH3), 4.42 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.79 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.41 (2H, s, -OCH2CN-), 6.19–6.21 (1H, m, ArH), 6.74–6.80 (4H, m, ArH), 6.78 (1H, s, -C=CH), 6.87 (2H, d, J = 8.8 Hz, ArH), 7.11 (1H, d, J = 8.4 Hz, ArH), 7.27 (1H, d, J = 15.2 Hz, -CH=CHCO-, overlain by CDCl3), 7.58 (1H, dd, J = 8.4, 1.6 Hz, ArH), 7.64 (1H, d, J = 2.0 Hz, ArH), 7.69 (1H, d, J = 8.8 Hz, ArH), 7.78 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.82 (2H, d, J = 8.8 Hz, ArH), 7.87 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 34.3, 49.7, 56.0, 56.0, 62.8, 66.2, 96.6, 109.6, 111.0, 111.4, 112.0, 112.1, 112.3, 114.8, 114.9, 116.2, 122.3, 124.3, 125.6, 126.1, 127.6, 130.2, 131.6, 132.4, 133.1, 143.8, 147.0, 149.4, 151.2, 158.6, 167.2, 168.2, 182.8, 188.0. LCMS (ES+) m/z = 633.3 ([M + H]+, tR = 1.94 min). HRMS (ESI+) m/z = 633.2352 [M + H]+ found, C36H33N4O7+ required 633.2349.
2-((Z)-4-(2-(4-((4-Bromo-2-((E)-3-(1-methyl-1H-indol-3-yl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)benzylidene)benzofuran-3(2H)-one (119). A mixture of alkyne chalcone 21 (308 mg, 0.781 mmol), azide aurone 88 (239 mg, 0.779 mmol), CuSO4·5H2O (261 mg, 1.04 mmol) and sodium ascorbate (397 mg, 2.00 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 119 (117 mg, 21%) as a pale yellow-brown powdery solid. m.p. 138–140 °C. TLC Rf = 0.44 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2930w (C-H str), 1699m (C=O str), 1644m, 1590s (C=C str), 1525m (C=C str), 1508m (C=C str), 1460m, 1395w, 1373m, 1249m, 1177s, 1126m, 1096w, 1045w. 1H-NMR (500 MHz, CDCl3): δ 3.74 (3H, s, -NCH3), 4.03 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.37 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.32 (2H, s, -OCH2CN-), 6.71 (2H, d, J = 8.8 Hz, ArH), 6.82 (1H, s, -C=CH), 7.04 (1H, d, J = 8.8 Hz, ArH), 7.16–7.21 (1H, m, ArH), 7.23 (1H, t, J = 7.6 Hz, ArH), 7.29–7.30 (2H, m, ArH), 7.33–7.35 (2H, m, ArH), 7.38 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.54 (1H, dd, J = 8.8, 2.4 Hz, ArH), 7.64–7.69 (2H, m, ArH), 7.73 (1H, s, -CHN-), 7.77 (2H, d, J = 8.8 ArH), 7.80–7.82 (2H, m, ArH), 7.85 (1H, d, J = 15.6 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 33.2, 49.2, 63.3, 65.7, 110.0, 112.7, 112.7, 112.9, 114.0, 114.7, 114.9, 120.6, 121.7, 121.7, 121.8, 123.1, 123.4, 124.1, 124.6, 125.7, 125.8, 132.0, 133.1, 133.2, 134.8, 135.0, 136.7, 138.1, 138.1, 143.7, 146.0, 155.5, 158.8, 165.8, 184.5, 190.8. LCMS (ES+) m/z = 703.1 ([M + H]+, tR = 5.05 min). HRMS (ESI+) m/z = 723.1189 [M + Na]+ found, C38H29O5N4BrNa+ required 723.1214.
(Z)-3-((1-(2-(4-((6-Methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-2-phenyl-4H-chromen-4-one (120). A mixture of alkyne flavone 30 (250 mg, 0.906 mmol), azide aurone 89 (316 mg, 0.936 mmol), CuSO4·5H2O (284 mg, 1.14 mmol) and sodium ascorbate (447 mg, 2.26 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 120 (351 mg, 63%) as a pale yellow-white powdery solid. m.p. 186–188 °C. TLC Rf = 0.38 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3056w (C-H str), 1698m (C=O str), 1654w, 1608s, 1595s (C=C str), 1509m (C=C str), 1470m, 1434m, 1397m, 1342m, 1272s, 1245s, 1198s, 1176m, 1147m, 1128s, 1109s, 1091s, 1042m. 1H-NMR (500 MHz, CDCl3): δ 3.93 (3H, s, -OCH3), 4.35 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.71 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.33 (2H, s, -OCH2CN-), 6.74–6.77 (3H, m, ArH and -C=CH), 6.90 (2H, d, J = 9.0 Hz, ArH), 7.40–7.45 (4H, m, ArH), 7.52 (1H, dd, J = 8.5, 0.5 Hz, ArH), 7.69 (1H, t, J = 8.5 Hz, ArH), 7.70 (1H, d, J = 8.5 Hz, ArH), 7.83 (2H, d, J = 9.0 Hz, ArH), 7.83 (1H, s, -CHN-), 8.01–8.03 (2H, m, ArH), 8.28 (1H, dd, J = 8.0, 1.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.5, 56.0, 65.1, 66.2, 96.6, 111.5, 112.0, 114.9, 115.0, 118.1, 124.1, 124.8, 124.9, 125.7, 126.1, 128.3, 128.7, 130.7, 133.1, 133.5, 139.5, 144.0, 147.0, 155.3, 156.4, 158.8, 167.3, 168.3, 175.1, 182.8. LCMS (ES+) m/z = 614.2 ([M + H]+, tR = 1.91 min). HRMS (ESI+) m/z = 614.1926 [M + H]+ found, C36H28N3O7+ required 614.1927.
(Z)-6-((1-(2-(4-((6-Methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-phenyl-4H-chromen-4-one (121). A mixture of alkyne flavone 31 (249 mg, 0.900 mmol), azide aurone 89 (309 mg, 0.916 mmol), CuSO4·5H2O (247 mg, 0.989 mmol) and sodium ascorbate (482 mg, 2.43 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 121 (269 mg, 49%) as a bright yellow-orange powdery solid. m.p. 148–150 °C. TLC Rf = 0.30 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3065w (C-H str), 2925w (C-H str), 1695m (C=O str), 1640s, 1594s (C=C str), 1568s (C=C str), 1510m (C=C str), 1496m, 1481m, 1454s, 1442s, 1360m, 1270m, 1250s, 1181s, 1131s, 1110s, 1095s, 1043m, 1027m. 1H-NMR (500 MHz, CDCl3): δ 3.92 (3H, s, -OCH3), 4.43 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.82 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.30 (2H, s, -OCH2CN-), 6.72–6.74 (2H, m, ArH), 6.75 (1H, s, -C=CH), 6.79 (1H, s, -C=CH), 6.89 (2H, d, J = 8.8 Hz, ArH), 7.33 (1H, dd, J = 9.2, 3.2 Hz, ArH), 7.48–7.52 (4H, m, ArH), 7.68 (1H, d, J = 9.2 Hz, ArH), 7.71 (1H, d, J = 3.2 Hz, ArH), 7.80 (2H, d, J = 8.8 Hz, ArH), 7.88–7.90 (3H, m, ArH and -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.0, 62.2, 66.3, 96.5, 106.4, 106.8, 111.4, 112.0, 114.8, 114.9, 120.0, 123.9, 124.2, 124.5, 125.7, 126.1, 126.2, 129.0, 131.5, 131.7, 133.1, 143.4, 146.9, 151.2, 155.4, 158.7, 163.2, 167.2, 168.2, 178.1, 182.8. LCMS (ES+) m/z = 614.2 ([M + H]+, tR = 1.77 min). HRMS (ESI+) m/z = 614.1906 [M + H]+ found, C36H28O7N3+ required 614.1922.
(Z)-3-Hydroxy-2-(4-((1-(2-(4-((3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-4H-chromen-4-one (122). A mixture of alkyne flavonol 36 (391 mg, 1.34 mmol), azide aurone 88 (408 mg, 1.33 mmol), CuSO4·5H2O (435 mg, 1.74 mmol) and sodium ascorbate (682 mg, 3.44 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 122 (335 mg, 42%) as a yellow-brown powdery solid. m.p. 208–210 °C. TLC Rf = 0.32 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3300w (O-H str), 3073w (C-H str), 2951w (C-H str), 1694m (C=O str), 1646w, 1604s, 1596s (C=C str), 1567w (C=C str), 1509s (C=C str), 1458w, 1426w, 1407w, 1299m, 1256s, 1178s, 1109s, 1051s. 1H-NMR (500 MHz, CDCl3): δ 4.46 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.34 (2H, s, -OCH2CN-), 6.82 (1H, s, -C=CH), 6.92 (2H, d, J = 8.8 Hz, ArH), 6.96 (1H, br s, OH), 7.15 (2H, d, J = 8.8 Hz, ArH), 7.22 (1H, t, J = 7.6 Hz, ArH), 7.31 (1H, d, J = 8.4 Hz, ArH), 7.39 (1H, t, J = 7.6 Hz, ArH), 7.56 (1H, d, J = 8.8 Hz, ArH), 7.63–7.71 (2H, m, ArH), 7.80 (1H, d, J = 7.6 Hz, ArH), 7.86 (3H, d, J = 8.4 Hz, ArH and -CHN-), 8.21–8.25 (3H, m, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 62.1, 66.4, 112.6, 112.9, 114.9, 114.9, 118.1, 120.6, 121.8, 123.4, 124.1, 124.4, 124.6, 125.4, 126.1, 129.5, 133.0, 133.4, 136.5, 136.7, 137.7, 145.0, 146.1, 155.3, 157.4, 158.9, 159.5, 165.9, 173.1, 184.5. LCMS (ES+) m/z = 600.0 ([M + H]+, tR = 4.68 min). HRMS (ESI+) m/z = 600.1751 [M + H]+ found, C35H26O7N3+ required 600.1765.
(Z)-2-(4-(2-(4-((3-((4,6-Dimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-hydroxy-7-methoxy-4H-chromen-4-one (123). A mixture of alkyne aurone 56 (192 mg, 0.571 mmol), azide flavonol 80 (210 mg, 0.595 mmol), CuSO4·5H2O (234 mg, 0.939 mmol) and sodium ascorbate (362 mg, 1.83 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 123 (54.3 mg, 14%) as a pale yellow-brown powdery solid. m.p. 178–180 °C. TLC Rf = 0.29 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3302w (O-H str), 2939w (C-H str), 2845w (C-H str), 1692w, 1614s (C=O str), 1599s (C=C str), 1510m (C=C str), 1503m (C=C str), 1452m, 1403w, 1361w, 1346w, 1251m, 1215s, 1187w, 1156m, 1121w, 1092s, 1036w. 1H-NMR (500 MHz, CDCl3): δ 3.91 (3H, s, -OCH3), 3.92 (3H, s, -OCH3), 3.95 (3H, s, -OCH3), 4.46 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 4.83 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.34 (2H, s, -OCH2CN-), 6.08 (1H, s, ArH), 6.41 (1H, s, ArH), 6.70 (1H, s, -C=CH), 6.93–6.95 (4H, m, ArH and OH), 6.99–7.03 (2H, m, ArH), 7.34 (1H, t, J = 8.0 Hz, ArH), 7.40 (1H, d, J = 7.5 Hz, ArH), 7.55 (1H, s, ArH), 7.85 (1H, br s, -CHN-), 8.12 (1H, d, J = 9.0 Hz, ArH), 8.16 (2H, d, J = 8.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.9, 56.2, 56.2, 62.2, 66.4, 89.3, 94.1, 99.9, 105.1, 110.3, 114.4, 114.6, 114.8, 116.2, 116.7, 121.9, 123.9, 124.5, 124.8, 126.7, 129.3, 129.8, 134.0, 137.4, 144.0, 148.0, 157.2, 158.3, 158.8, 159.3, 164.2, 169.1, 172.6, 180.6. LCMS (ES+) m/z = 690.2 ([M + H]+, tR = 4.59 min). HRMS (ESI+) m/z = 690.2062 [M + H]+ found, C38H32O10N3+ required 690.2082.
(Z)-7-((1-(2-(4-((6-Methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-methoxy)-3-(4-methoxyphenyl)-4H-chromen-4-one (124). A mixture of alkyne isoflavone 46 (276 mg, 0.901 mmol), azide aurone 89 (301 mg, 0.892 mmol), CuSO4·5H2O (348 mg, 1.39 mmol) and sodium ascorbate (496 mg, 2.50 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 124 (317 mg, 55%) as a bright yellow powdery solid. m.p. 228–230 °C. TLC Rf = 0.43 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3085w (C-H str), 2935w (C-H str), 1705m (C=O str), 1651m, 1629s, 1596s (C=C str), 1567m (C=C str), 1510s (C=C str), 1441s, 1347m, 1295m, 1249s, 1202m, 1179s, 1147s, 1098s, 1020s. 1H-NMR (500 MHz, CDCl3): δ 3.83 (3H, s, -OCH3), 3.93 (3H, s, -OCH3), 4.44 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.83 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.34 (2H, s, -OCH2CN-), 6.74–6.77 (2H, m, ArH), 6.76 (1H, s, -C=CH), 6.88 (2H, d, J = 8.8 Hz, ArH), 6.93 (2H, d, J = 8.8 Hz, ArH), 6.97 (1H, d, J = 2.4 Hz, ArH), 7.06 (1H, dd, J = 8.8, 2.4 Hz, ArH), 7.47 (2H, d, J = 8.8 Hz, ArH), 7.69 (1H, d, J = 8.4 Hz, ArH), 7.82 (2H, d, J = 8.8 Hz, ArH), 7.87 (1H, s, -CHN-), 7.89 (1H, s, -C=CH), 8.23 (1H, d, J = 8.8 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 55.3, 56.0, 62.4, 66.3, 96.7, 101.3, 111.3, 112.1, 113.9, 114.8, 114.9, 118.8, 124.1, 124.2, 124.9, 125.7, 126.3, 127.9, 130.0, 133.1, 143.2, 147.0, 152.1, 157.7, 158.6, 159.5, 162.3, 167.3, 168.3, 175.8, 182.8. LCMS (ES+) m/z = 644.2 ([M + H]+, tR = 1.86 min). HRMS (ESI+) m/z = 644.2011 [M + H]+ found, C37H30O8N3+ required 644.2027.
4,6-Dimethoxy-2-((Z)-3-((1-(2-(4-(((Z)-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)benzofuran-3(2H)-one (125). A mixture of alkyne aurone 56 (303 mg, 0.902 mmol), azide aurone 88 (278 mg, 0.905 mmol), CuSO4·5H2O (263 mg, 1.05 mmol) and sodium ascorbate (462 mg, 2.33 mmol) in t-BuOH/H2O (1:1, 20 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 125 (491 mg, 85%) as a bright yellow-brown powdery solid. m.p. 148–150 °C. TLC Rf = 0.36 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2942w (C-H str), 2843w (C-H str), 1694m (C=O str), 1651m, 1587s (C=C str), 1508m (C=C str), 1458m, 1423w, 1345m, 1298m, 1251m, 1234m, 1214s, 1177s, 1154s, 1128s, 1091s, 1036m. 1H-NMR (500 MHz, CDCl3): δ 3.90 (3H, s, -OCH3), 3.92 (3H, s, -OCH3), 4.42 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.81 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.31 (2H, s, -OCH2CN-), 6.09 (1H, d, J = 1.6 Hz, ArH), 6.40 (1H, d, J = 1.2 Hz, ArH), 6.68 (1H, s, -C=CH), 6.81 (1H, s, -C=CH), 6.88 (2H, d, J = 8.4 Hz, ArH), 7.00 (1H, dd, J = 8.0, 2.4 Hz, ArH), 7.21 (1H, t, J = 7.6 Hz, ArH), 7.30–7.34 (2H, m, ArH), 7.39 (1H, d, J = 8.0 Hz, ArH), 7.54 (1H, s, ArH), 7.64 (1H, t, J = 7.6 Hz, ArH), 7.79 (1H, d, J = 7.6 Hz, ArH), 7.82–7.85 (3H, m, ArH and -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.2, 62.2, 66.3, 89.3, 94.1, 105.1, 110.3, 112.7, 112.9, 114.3, 114.8, 116.3, 116.6, 121.8, 123.3, 123.9, 124.5, 124.5, 126.0, 129.8, 133.0, 133.4, 133.9, 136.7, 144.3, 146.1, 148.0, 158.3, 158.9, 159.3, 165.9, 169.0, 180.6, 184.5. LCMS (ES+) m/z = 644.2 ([M + H]+, tR = 2.02 min). HRMS (ESI+) m/z = 644.2006 [M + H]+ found, C37H30O8N3+ required 644.2027.
(E)-4-((1-(2-(4-(3-(2-Hydroxyphenyl)-3-oxoprop-1-en-1-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (126). A mixture of alkyne coumarin 48 (207 mg, 1.03 mmol), azide chalcone 61 (302 mg, 0.975 mmol), CuSO4·5H2O (292 mg, 1.17 mmol) and sodium ascorbate (508 mg, 2.56 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 126 (387 mg, 78%) as a bright yellow powdery solid. m.p. 194–196 °C. TLC Rf = 0.43 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3083w (C-H str), 2929w (C-H str), 1725s (C=O str), 1641m, 1625m, 1607m, 1560s (C=C str), 1511s (C=C str), 1489s, 1424m, 1382m, 1273m, 1249s, 1202s, 1175s, 1156s, 1107m, 1056m, 1030m. 1H-NMR (500 MHz, CDCl3): δ 4.47 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.87 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.36 (2H, s, -OCH2CN-), 5.87 (1H, s, -C=CH), 6.91–6.97 (3H, m, ArH), 7.03 (1H, d, J = 8.4 Hz, ArH), 7.23 (1H, t, J = 8.0 Hz, ArH), 7.31 (1H, d, J = 8.4 Hz, ArH), 7.48–7.54 (2H, m, ArH), 7.54 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.61 (2H, d, J = 8.4 Hz, ArH), 7.78 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.86 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.91 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.94 (1H, s, -CHN-), 12.87 (1H, s, OH). 13C-NMR (500 MHz, CDCl3): δ 49.9, 62.5, 66.3, 91.2, 115.0, 115.4, 116.8, 118.4, 118.6, 118.8, 120.0, 123.1, 123.9, 124.7, 128.5, 129.5, 130.6, 132.6, 136.3, 141.6, 144.7, 153.3, 159.8, 162.6, 163.5, 164.9, 193.6. LCMS (ES+) m/z = 510.2 ([M + H]+, tR = 1.65 min). HRMS (ESI+) m/z = 510.1651 [M + H]+ found, C29H24O6N3+ required 510.1660.
4-((1-(2-(4-(3-Hydroxy-4-oxo-4H-chromen-2-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (127). A mixture of alkyne coumarin 48 (186 mg, 0.931 mmol), azide flavonol 79 (302 mg, 0.933 mmol), CuSO4·5H2O (318 mg, 1.27 mmol) and sodium ascorbate (497 mg, 2.51 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 127 (77.9 mg, 16%) as an off-white powdery solid. m.p. 228–230 °C. TLC Rf = 0.46 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3271w (O-H str), 3015w (C-H str), 2923w (C-H str), 1714s (C=O str), 1622m, 1603s, 1565s (C=C str), 1509m (C=C str), 1480m, 1470w, 1428m, 1406w, 1376w, 1262s, 1228m, 1177m, 1140w, 1106m, 1043m. 1H-NMR (500 MHz, CDCl3): δ 4.52 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.89 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.37 (2H, s, -OCH2CN-), 5.89 (1H, s, -C=CH), 6.97 (1H, br s, OH), 7.03 (2H, d, J = 9.0 Hz, ArH), 7.24 (1H, t, J = 8.0 Hz, ArH), 7.31 (1H, dd, J = 8.5, 1.0 Hz, ArH), 7.43 (1H, t, J = 8.0 Hz, ArH), 7.54 (1H, t, J = 8.5 Hz, ArH), 7.59 (1H, d, J = 8.5 Hz, ArH), 7.72 (1H, t, J = 8.5 Hz, ArH), 7.78 (1H, dd, J = 8.0, 1.5 Hz, ArH), 7.96 (1H, s, -CHN-), 8.23–8.27 (3H, m, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.9, 62.6, 66.3, 91.2, 114.5, 115.4, 116.8, 118.2, 120.7, 123.1, 123.9, 124.5, 124.7, 124.8, 125.4, 129.7, 132.5, 133.5, 137.8, 141.7, 144.6, 153.3, 155.3, 159.0, 162.6, 164.9, 173.2. LCMS (ES+) m/z = 524.2 ([M + H]+, tR = 1.55 min). HRMS (ESI+) m/z = 524.1476 [M + H]+ found, C29H22N3O7+ required 524.1458.
4-(2-(4-(((4-Oxo-2-phenyl-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)-2H-chromen-2-one (128). A mixture of alkyne flavone 30 (310 mg, 1.12 mmol), azide coumarin 58 (256 mg, 1.11 mmol), CuSO4·5H2O (399 mg, 1.60 mmol) and sodium ascorbate (578 mg, 2.92 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 128 (354 mg, 63%) as a white fluffy solid. m.p. 268–270 °C. TLC Rf = 0.30 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3082w (C-H str), 2926w (C-H str), 1723s (C=O str), 1626s, 1567m (C=C str), 1494w, 1465m, 1400w, 1385m, 1274m, 1239s, 1197s, 1182s, 1148s, 1140m, 1111m, 1058w, 1030w. 1H-NMR (500 MHz, CDCl3): δ 4.47 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.85 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 5.31 (2H, s, -OCH2CN-), 5.65 (1H, s, -C=CH), 7.24 (1H, t, J = 7.5 Hz, ArH, overlain by CDCl3), 7.33 (1H, d, J = 8.5 Hz, ArH), 7.41–7.46 (4H, m, ArH), 7.51 (1H, d, J = 8.5 Hz, ArH), 7.55 (1H, t, J = 8.0 Hz, ArH), 7.68–7.73 (2H, m, ArH), 7.93 (1H, s, -CHN-), 8.02–8.04 (2H, m, ArH), 8.25 (1H, d, J = 7.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 48.7, 65.2, 67.0, 91.1, 115.0, 116.8, 118.1, 122.9, 124.0, 124.2, 124.7, 124.9, 125.6, 128.4, 128.7, 130.7, 130.8, 132.8, 133.7, 139.7, 144.7, 153.3, 155.3, 156.3, 162.2, 164.6, 175.1. LCMS (ES+) m/z = 508.3 ([M + H]+, tR = 1.81 min). HRMS (ESI+) m/z = 508.1506 [M + H]+ found, C29H22N3O6+ required 508.1509.
(Z)-4-((1-(2-(4-((6-Methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (129). A mixture of alkyne coumarin 48 (181 mg, 0.902 mmol), azide aurone 89 (302 mg, 0.895 mmol), CuSO4·5H2O (250 mg, 1.00 mmol) and sodium ascorbate (459 mg, 2.32 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and recrystallized from MeOH to afford triazole hybrid 129 (253 mg, 53%) as a bright yellow-orange microcrystalline solid. m.p. 138–140 °C. TLC Rf = 0.40 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3077w (C-H str), 2926w (C-H str), 1719s (C=O str), 1650m, 1623m, 1593s (C=C str), 1565m (C=C str), 1511m (C=C str), 1442m, 1399m, 1269m, 1247s, 1182s, 1130s, 1096s, 1042m, 1019m. 1H-NMR (500 MHz, CDCl3): δ 3.94 (3H, s, -OCH3), 4.48 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 4.87 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.36 (2H, s, -OCH2CN-), 5.88 (1H, s, -C=CH), 6.75–6.78 (2H, m, ArH), 6.76 (1H, s, -C=CH), 6.94 (2H, d, J = 9.0 Hz, ArH), 7.23 (1H, t, J = 8.0 Hz, ArH), 7.31 (1H, dd, J = 8.5, 1.0 Hz, ArH), 7.53 (1H, t, J = 8.5 Hz, ArH), 7.71 (1H, d, J = 9.0 Hz, ArH), 7.77 (1H, dd, J = 8.0, 1.5 Hz, ArH), 7.84 (2H, d, J = 8.5 Hz, ArH), 7.95 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.9, 56.0, 62.5, 66.3, 91.2, 96.6, 111.3, 112.1, 114.8, 115.0, 115.4, 116.8, 123.1, 123.9, 124.7, 125.8, 126.3, 132.5, 133.1, 141.6, 147.1, 153.3, 158.6, 162.6, 164.9, 167.3, 168.3, 182.8. LCMS (ES+) m/z = 538.2 ([M + H]+, tR = 1.78 min). HRMS (ESI+) m/z = 538.1617 [M + H]+ found, C30H24N3O7+ required 538.1614.
4-(2-(4-(((3-(4-Methoxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)-2H-chromen-2-one (130). A mixture of alkyne isoflavone 46 (301 mg, 0.983 mmol), azide coumarin 58 (232 mg, 1.00 mmol), CuSO4·5H2O (288 mg, 1.15 mmol) and sodium ascorbate (503 mg, 2.54 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 130 (363 mg, 69%) as an off-white powdery solid. m.p. 208–210 °C. TLC Rf = 0.28 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3085w (C-H str), 2927w (C-H str), 1730s (C=O str), 1621s, 1566m (C=C str), 1513m (C=C str), 1495w, 1444m, 1379m, 1330w, 1279w, 1241s, 1198m, 1181s, 1144m, 1109m, 1031m. 1H-NMR (500 MHz, CDCl3): δ 3.85 (3H, s, -OCH3), 4.58 (2H, t, J = 4.4 Hz, -OCH2CH2N-), 4.95 (2H, t, J = 4.4 Hz, -OCH2CH2N-), 5.35 (2H, s, -OCH2CN-), 5.69 (1H, s, -C=CH), 6.97–6.99 (3H, m, ArH), 7.03 (1H, dd, J = 8.8, 2.0 Hz, ArH), 7.23 (1H, d, J = 7.6 Hz, ArH), 7.31 (1H, d, J = 8.0 Hz, ArH), 7.50 (2H, d, J = 8.4 Hz, ArH), 7.56 (1H, t, J = 8.0 Hz, ArH), 7.65 (1H, d, J = 8.0 Hz, ArH), 7.84 (1H, s, -CHN-), 7.90 (1H, s, -C=CH), 8.20 (1H, d, J = 8.8 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.0, 55.3, 62.4, 67.0, 91.3, 101.2, 114.0, 114.8, 114.9, 117.0, 118.9, 120.2, 122.4, 123.5, 124.0, 124.1, 125.0, 128.0, 130.1, 132.9, 143.7, 152.1, 153.3, 157.7, 159.6, 162.2, 164.4, 175.7. LCMS (ES+) m/z = 538.0 ([M + H]+, tR = 4.19 min). HRMS (ESI+) m/z = 538.1624 [M + H]+ found, C30H24N3O7+ required 538.1614.
(E)-4-((1-(2-(4-(3-Oxo-3-(2-(prop-2-yn-1-yloxy)phenyl)prop-1-en-1-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (131). A mixture of biflavonoid 126 (264 mg, 0.517 mmol), propargyl bromide (0.130 mL, 1.46 mmol) and anhydrous K2CO3 (291 mg, 2.10 mmol) in dry acetone (50 mL) was reacted according to GP-B. The crude residue was purified by flash column chromatography (SiO2, 1% MeOH/CH2Cl2) to afford alkyne biflavonoid 131 (229 mg, 81%) as a pale yellow-white powdery solid. m.p. 164–166 °C. TLC Rf = 0.15 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3239w (C≡C-H str), 3074w (C-H str), 2973w (C-H str), 2165w (C≡C str), 1723s (C=O str), 1651m, 1624m, 1600s (C=C str), 1566m (C=C str), 1510m (C=C str), 1482w, 1452m, 1401s, 1372m, 1328m, 1231s, 1175w, 1105m, 1028m. 1H-NMR (500 MHz, CDCl3): δ 2.54 (1H, t, J = 2.0 Hz, -OCH2C≡CH), 4.45 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.79 (2H, d, J = 2.0 Hz, -OCH2C≡CH), 4.85 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.35 (2H, s, -OCH2CN-), 5.87 (1H, s, -C=CH), 6.88 (2H, d, J = 8.8 Hz, ArH), 7.09–7.13 (2H, m, ArH), 7.23 (1H, t, J = 7.6 Hz, ArH), 7.29 (1H, d, J = 13.2 Hz, -CH=CHCO-, overlain by CDCl3), 7.32 (1H, t, J = 4.4 Hz, ArH), 7.49 (1H, t, J = 8.8 Hz, ArH), 7.53–7.58 (3H, m, ArH), 7.56 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.64 (1H, dd, J = 7.6, 1.2 Hz, ArH), 7.77 (1H, dd, J = 8.4, 0.8 Hz, ArH), 7.93 (1H, s, -CHN-). 13C-NMR (500 MHz, CDCl3): δ 49.9, 56.4, 62.5, 66.3, 76.1, 78.1, 91.2, 113.3, 114.8, 115.4, 116.8, 121.8, 123.1, 123.9, 124.6, 125.6, 129.0, 130.0, 130.2, 130.5, 132.6, 132.6, 141.6, 142.7, 153.3, 155.8, 159.3, 162.5, 164.9, 192.5. LCMS (ES+) m/z = 548.0 ([M + H]+, tR = 4.38 min). HRMS (ESI+) m/z = 548.1803 [M + H]+ found, C32H26O6N3+ required 548.1816.
(Z)-2-(4-((1-(2-(4-((3-Oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-3-(prop-2-yn-1-yloxy)-4H-chromen-4-one (132). A mixture of biflavonoid 122 (212 mg, 0.354 mmol), propargyl bromide (0.063 mL, 0.707 mmol) and anhydrous K2CO3 (153 mg, 1.11 mmol) in dry acetone (20 mL) was reacted according to GP-B. The crude residue was purified by flash column chromatography (SiO2, 1% MeOH/CH2Cl2) to afford alkyne biflavonoid 132 (168 mg, 74%) as a bright yellow powdery solid. m.p. 150–152 °C. TLC Rf = 0.50 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3233w (C≡C-H str), 2939w (C-H str), 2115w (C≡C str), 1697s (C=O str), 1635m, 1606s, 1598s (C=C str), 1566m (C=C str), 1508s (C=C str), 1472m, 1395m, 1343w, 1301m, 1250s, 1198m, 1184s, 1148m, 1133w, 1047m, 1017w. 1H-NMR (500 MHz, CDCl3): δ 2.34 (1H, t, J = 2.4 Hz, -OCH2C≡CH), 4.46 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.00 (2H, d, J = 2.4 Hz, -OCH2C≡CH), 5.33 (2H, s, -OCH2CN-), 6.83 (1H, s, -C=CH), 6.93 (2H, d, J = 8.8 Hz, ArH), 7.12 (2H, d, J = 9.2 Hz, ArH), 7.22 (1H, t, J = 7.6 Hz, ArH), 7.31 (1H, d, J = 8.4 Hz, ArH), 7.38 (1H, t, J = 7.6 Hz, ArH), 7.51 (1H, d, J = 8.4 Hz, ArH), 7.63–7.69 (2H, m, ArH), 7.80 (1H, d, J = 7.6 Hz, ArH), 7.86 (2H, d, J = 6.8 Hz, ArH), 7.87 (1H, s, -CHN-), 8.16 (2H, d, J = 8.8 Hz, ArH), 8.22 (1H, dd, J = 8.0, 1.6 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.8, 59.0, 62.0, 66.3, 76.0, 78.7, 112.6, 112.9, 114.6, 114.9, 117.9, 121.8, 123.4, 123.8, 123.9, 124.1, 124.6, 124.7, 125.7, 126.1, 130.7, 133.4, 136.7, 138.0, 146.1, 155.1, 156.4, 158.9, 160.1, 165.8, 174.7, 184.5. LCMS (ES+) m/z = 638.1 ([M + H]+, tr = 4.82 min). HRMS (ESI+) m/z = 638.1902 [M + H]+ found, C38H28O7N3+ required 638.1922.
(E)-1-(4-Methoxy-2-(prop-2-yn-1-yloxy)phenyl)-3-(4-methoxy-3-((1-(2-(2-methoxy-4-((E)-3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-2-en-1-one (133). A mixture of biflavonoid 92 (111 mg, 0.148 mmol), propargyl bromide (0.050 mL, 0.561 mmol) and anhydrous K2CO3 (113 mg, 0.815 mmol) in dry acetone (50 mL) was reacted according to GP-B. The crude residue was purified by flash column chromatography (SiO2, 1% MeOH/CH2Cl2) to afford alkyne biflavonoid 133 (107 mg, 92%) as a bright yellow powdery solid. m.p. 170–172 °C. TLC Rf = 0.29 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3286w (C≡C-H str), 3002w (C-H str), 2936w (C-H str), 2841w (C-H str), 2160w (C≡C str), 1650m (C=O str), 1599s (C=C str), 1511m (C=C str), 1458m, 1441w, 1432w, 1418w, 1399w, 1377w, 1337m, 1321m, 1301m, 1259s, 1205w, 1159m, 1124m, 1027m. 1H-NMR (500 MHz, CDCl3): δ 2.64 (1H, t, J = 2.4 Hz, -OCH2C≡CH), 3.87 (6H, s, 2 × -OCH3), 3.89 (3H, s, -OCH3), 3.91 (9H, s, 3 × -OCH3), 4.45 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.83 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.84 (2H, d, J = 2.4 Hz, -OCH2C≡CH), 5.33 (2H, s, -OCH2CN-), 6.15 (2H, s, ArH), 6.61–6.63 (2H, m, ArH), 6.83 (1H, d, J = 8.0 Hz, ArH), 6.87 (1H, d, J = 8.0 Hz, ArH), 7.18 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.39 (1H, d, J = 1.6 Hz, ArH), 7.46 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.56–7.64 (2H, m, ArH), 7.61 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.79 (1H, d, J = 9.2 Hz, ArH), 7.85 (1H, d, J = 15.6 Hz, -CH=CHCO-), 8.03 (1H, s, -CHN-), 8.23 (1H, d, J = 16.0 Hz, -CH=CHCO-). 13C-NMR (500 MHz, CDCl3): δ 49.7, 55.6, 55.8, 55.9, 55.9, 56.5, 63.1, 67.5, 76.3, 78.1, 90.5, 100.1, 106.4, 106.6, 111.5, 111.7, 112.4, 112.8, 121.6, 122.3, 122.8, 124.0, 124.6, 125.3, 128.5, 132.9, 133.7, 135.6, 142.1, 144.1, 147.7, 149.5, 150.6, 151.5, 158.2, 158.3, 161.6, 163.0, 163.8, 190.0, 190.3. LCMS (ES+) m/z = 790.3 ([M + H]+, tR = 4.71 min). HRMS (ESI+) m/z = 790.2949 [M + H]+ found, C44H44O11N3+ required 790.2970.
2-(4-(2-(4-(((4-Oxo-2-phenyl-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-(prop-2-yn-1-yloxy)-4H-chromen-4-one (134). A mixture of biflavonoid 110 (161 mg, 0.268 mmol), propargyl bromide (0.045 mL, 0.500 mmol) and anhydrous K2CO3 (111 mg, 0.803 mmol) in dry acetone (20 mL) was reacted according to GP-B. The crude residue was purified by flash column chromatography (SiO2, 1% MeOH/CH2Cl2) to afford alkyne biflavonoid 134 (161 mg, 94%) as a pale yellow-white powdery solid. m.p. 118–120 °C. TLC Rf = 0.34 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3233w (C≡C-H str), 2939w (C-H str), 2116w (C≡C str), 1631s (C=O str), 1613s, 1600s, 1559m (C=C str), 1508m (C=C str), 1467s, 1392s, 1287w, 1252m, 1239m, 1184s, 1146m, 1123w, 1109w, 1042w. 1H-NMR (500 MHz, CDCl3): δ 2.33 (1H, t, J = 2.4 Hz, -OCH2C≡CH), 4.39 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.73 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.99 (2H, d, J = 2.4 Hz, -OCH2C≡CH), 5.33 (2H, s, -OCH2CN-) 6.97 (2H, d, J = 9.2 Hz, ArH), 7.39–7.45 (5H, m, ArH), 7.53 (1H, d, J = 8.0 Hz, ArH), 7.54 (1H, d, J = 8.0 Hz, ArH), 7.67–7.72 (2H, m, ArH), 7.85 (1H, s, -CHN-), 8.02–8.04 (2H, m, ArH), 8.15 (2H, d, J = 9.2 Hz, ArH), 8.25 (1H, dd, J = 8.0, 1.2 Hz, ArH), 8.28 (1H, dd, J = 8.0, 1.2 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.5, 59.0, 65.1, 66.2, 76.1, 78.6, 114.3, 117.9, 118.1, 124.0, 124.1, 124.3, 124.7, 124.8, 124.9, 125.7, 128.3, 128.7, 130.7, 130.8, 133.4, 133.6, 138.1, 139.5, 144.1, 155.1, 155.3, 156.2, 156.4, 159.6, 174.7, 175.1. LCMS (ES+) m/z = 638.1 ([M + H]+, tR = 4.70 min). HRMS (ESI+) m/z = 638.1905 [M + H]+ found, C38H28O7N3+ required 638.1922.
(E)-2-(4-(2-(4-((4-(3-(Ferrocenyl)acryloyl)-2-methoxyphenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-(prop-2-yn-1-yloxy)-4H-chromen-4-one (135). A mixture of biflavonoid 106 (190 mg, 0.263 mmol), propargyl bromide (0.047 mL, 0.526 mmol) and anhydrous K2CO3 (127 mg, 0.920 mmol) in dry acetone (20 mL) was reacted according to GP-B. The crude residue was purified by flash column chromatography (SiO2, 1% MeOH/CH2Cl2) to afford alkyne biflavonoid 135 (95.5 mg, 48%) as a dark red powdery solid. m.p. 108–110 °C. TLC Rf = 0.37 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3292w (C≡C-H str), 2937w (C-H str), 2245w (C≡C str), 1636m (C=O str), 1598s (C=C str), 1576s (C=C str), 1509s (C=C str), 1467m, 1419m, 1395m, 1349w, 1258s, 1198m, 1184m, 1150m, 1029w, 1000w. 1H-NMR (500 MHz, CDCl3): δ 2.33 (1H, t, J = 2.5 Hz, -OCH2C≡CH), 3.96 (3H, s, -OCH3), 4.17 (5H, s, -C5H5), 4.45–4.47 (4H, m, -C5H4 and -OCH2CH2N-), 4.57 (2H, t, J = 2.0 Hz, -C5H4), 4.82 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.98 (2H, d, J = 2.5 Hz, -OCH2C≡CH), 5.42 (2H, s, -OCH2CN-), 6.96 (2H, d, J = 9.0 Hz, ArH), 7.13 (1H, d, J = 15.5 Hz, -CH=CHCO-), 7.15 (1H, d, J = 8.5 Hz, ArH), 7.41 (1H, t, J = 8.5 Hz, ArH), 7.54 (1H, dd, J = 8.5, 0.5 Hz, ArH), 7.58 (1H, dd, J = 8.5, 2.0 Hz, ArH), 7.63 (1H, d, J = 2.0 Hz, ArH), 7.69 (1H, t, J = 8.5 Hz, ArH), 7.73 (1H, d, J = 15.0 Hz, -CH=CHCO-), 7.90 (1H, s, -CHN-), 8.14 (2H, d, J = 9.0 Hz, ArH), 8.25 (1H, dd, J = 8.0, 1.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.7, 56.1, 59.0, 62.8, 66.2, 68.9, 69.7, 71.2, 76.1, 78.6, 79.3, 111.2, 112.3, 114.2, 117.9, 118.5, 122.4, 124.0, 124.3, 124.4, 124.7, 125.7, 130.8, 132.3, 133.4, 138.1, 143.8, 146.0, 149.5, 151.3, 155.1, 156.1, 159.5, 174.7, 187.9. LCMS (ES+) m/z = 762.1 ([M + H]+, tR = 4.88 min). HRMS (ESI+) m/z = 762.1878 [M + H]+ found, C43H36O7N3Fe+ required 762.1897.
(E)-4-((1-(2-(4-(3-Oxo-3-(2-((1-(2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)prop-1-en-1-yl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2H-chromen-2-one (136). A mixture of alkyne biflavonoid 131 (205 mg, 0.375 mmol), azide flavone 86 (123 mg, 0.399 mmol), CuSO4·5H2O (115 mg, 0.459 mmol) and sodium ascorbate (192 mg, 0.969 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 136 (214 mg, 67%) as a pale yellow-white powdery solid. m.p. 78–80 °C. TLC Rf = 0.37 (7% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3080w (C-H str), 2923w (C-H str), 1708s (C=O str), 1622s, 1597s (C=C str), 1567s (C=C str), 1509w (C=C str), 1493w, 1449m, 1356m, 1274w, 1237m, 1173m, 1139w, 1105w, 1043w, 1026w. 1H-NMR (500 MHz, CDCl3): δ 4.38 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.41 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.70 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 4.82 (2H, t, J = 5.2 Hz, -OCH2CH2N-), 5.34 (2H, s, -OCH2CN-), 5.34 (2H, s, -OCH2CN-), 5.85 (1H, s, -C=CH), 6.75 (1H, s, -C=CH), 6.77–6.80 (3H, m, ArH), 6.85 (1H, d, J = 2.4 Hz, ArH), 7.08 (1H, t, J = 7.6 Hz, ArH), 7.14 (1H, d, J = 8.4 Hz, ArH), 7.20 (1H, t, J = 8.0 Hz, ArH), 7.26 (1H, d, J = 16.0 Hz, -CH=CHCO-, overlain by CDCl3), 7.28 (1H, d, J = 4.0 Hz, ArH), 7.38 (2H, d, J = 8.4 Hz, ArH), 7.46–7.54 (5H, m, ArH), 7.50 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.62 (1H, dd, J = 8.8, 1.6 Hz, ArH), 7.74 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.77 (1H, s, -CHN-), 7.89 (2H, dd, J = 7.6, 1.6 Hz, ArH), 7.96 (1H, s, -CHN-), 8.03 (1H, d, J = 8.8 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 49.8, 62.5, 62.8, 66.2, 66.6, 91.2, 101.4, 107.5, 113.1, 114.1, 114.8, 115.4, 116.7, 118.5, 121.5, 123.1, 123.9, 124.0, 124.8, 125.7, 126.2, 127.3, 128.7, 129.1, 129.8, 130.1, 130.4, 131.6, 131.6, 132.5, 132.9, 141.6, 142.4, 144.1, 153.3, 156.5, 157.6, 159.3, 161.9, 162.5, 163.2, 164.9, 177.6, 192.6. LCMS (ES+) m/z = 855.3 ([M + H]+, tR = 4.57 min). HRMS (ESI+) m/z = 855.2743 [M + H]+ found, C49H39O9N6+ required 855.2773.
(Z)-7-(2-(4-(((4-Oxo-2-(4-((1-(2-(4-((3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)-2-phenyl-4H-chromen-4-one (137). A mixture of alkyne biflavonoid 132 (145 mg, 0.227 mmol), azide flavone 86 (73.5 mg, 0.239 mmol), CuSO4·5H2O (70.1 mg, 0.281 mmol) and sodium ascorbate (129 mg, 0.651 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 137 (106 mg, 49%) as a bright yellow powdery solid. m.p. 198–200 °C. TLC Rf = 0.33 (7% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2931w (C-H str), 2874w (C-H str), 1698w, 1628s (C=O str), 1602s, 1594s (C=C str), 1508s (C=C str), 1466m, 1449m, 1423w, 1396w, 1374m, 1299w, 1288w, 1249s, 1178s, 1148w, 1128w, 1110w, 1096w, 1041m. 1H-NMR (500 MHz, CDCl3): δ 4.44–4.46 (4H, m, 2 × -OCH2CH2N-), 4.78 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.83 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.28 (2H, s, -OCH2CN-), 5.33 (2H, s, -OCH2CN-), 6.75 (1H, s, -C=CH), 6.81 (1H, s, -C=CH), 6.91–6.94 (4H, m, ArH), 7.04 (2H, d, J = 9.2 Hz, ArH), 7.21 (1H, d, J = 7.6 Hz, ArH), 7.30 (1H, d, J = 8.4 Hz, ArH), 7.37 (1H, d, J = 7.6 Hz, ArH), 7.47–7.53 (4H, m, ArH), 7.62–7.67 (2H, m, ArH), 7.79 (1H, d, J = 7.6 Hz, ArH), 7.84 (2H, d, J = 8.8 Hz, ArH), 7.87–7.90 (3H, m, -CHN- and ArH), 7.94 (1H, s, -CHN-), 8.04 (2H, d, J = 8.8 Hz, ArH), 8.11 (1H, d, J = 8.8 Hz, ArH), 8.23 (1H, dd, J = 8.0, 1.2 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.3, 49.7, 61.9, 65.0, 66.3, 66.7, 101.5, 107.6, 112.7, 112.9, 114.2, 114.7, 114.9, 117.9, 118.5, 121.8, 123.4, 123.6, 124.0, 124.1, 124.6, 124.7, 125.0, 125.6, 126.1, 126.2, 127.4, 129.0, 130.5, 131.5, 131.7, 133.4, 133.4, 136.7, 139.0, 143.7, 144.3, 146.1, 155.1, 156.1, 157.7, 159.0, 160.0, 162.1, 163.2, 165.9, 174.9, 177.7, 184.5. LCMS (ES+) m/z = 945.4 ([M + H]+, tR = 4.89 min). HRMS (ESI+) m/z = 945.2851 [M + H]+ found, C55H41O10N6+ required 945.2879.
7-Methoxy-2-(4-(2-(4-((5-methoxy-2-((E)-3-(4-methoxy-3-((1-(2-(2-methoxy-4-((E)-3-(2,4,6-trimethoxyphenyl)acryloyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)acryloyl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-4H-chromen-4-one (138). A mixture of alkyne biflavonoid 133 (107 mg, 0.135 mmol), azide flavone 87 (49.3 mg, 0.146 mmol), CuSO4·5H2O (66.0 mg, 0.264 mmol) and sodium ascorbate (72.8 mg, 0.367 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 138 (8.00 mg, 5%) as a pale yellow-white powdery solid. m.p. 128–130 °C. TLC Rf = 0.19 (5% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2927w (C-H str), 2846w (C-H str), 1736w, 1627m (C=O str), 1600s (C=C str), 1510s (C=C str), 1454m, 1439m, 1423m, 1375w, 1355w, 1338w, 1258s, 1204m, 1162m, 1123s, 1023s. 1H-NMR (500 MHz, DMSO-d6): δ 3.76 (6H, s, 2 × -OCH3), 3.85 (6H, s, 2 × -OCH3), 3.90 (9H, s, 3 × -OCH3), 4.38 (2H, t, J = 4.4 Hz, -OCH2CH2N-), 4.50 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.71 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 4.82 (2H, t, J = 4.8 Hz, -OCH2CH2N-), 5.24 (2H, s, -OCH2CN-), 5.38 (2H, s, -OCH2CN-), 6.29 (2H, s, ArH), 6.57–6.66 (1H, m, ArH), 6.81 (1H, s, -C=CH), 6.82–6.85 (1H, m, ArH), 6.92–6.98 (3H, m, ArH), 7.02–7.11 (3H, m, ArH), 7.20 (1H, d, J = 8.8 Hz, ArH), 7.27 (1H, dd, J = 5.6, 2.4 Hz, ArH), 7.47 (1H, d, J = 15.6 Hz, -CH=CHCO-), 7.48 (1H, d, J = 8.0 Hz, ArH), 7.54–7.64 (2H, m, ArH), 7.56 (1H, d, J = 15.2 Hz, -CH=CHCO-), 7.82 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.87–8.06 (3H, m, ArH and -CHN-), 8.02 (1H, d, J = 16.0 Hz, -CH=CHCO-), 8.28 (1H, d, J = 6.4 Hz, ArH), 8.31 (1H, s, -CHN-). 13C-NMR (500 MHz, DMSO-d6): δ 48.9, 49.0, 55.5, 55.7, 56.0, 61.5, 61.7, 66.2, 67.1, 91.0, 99.8, 100.9, 105.1, 105.4, 106.6, 111.0, 111.3, 111.8, 112.0, 112.7, 114.5, 114.9, 114.9, 115.0, 115.0, 117.1, 120.2, 122.1, 123.8, 125.3, 126.1, 127.6, 127.9, 128.0, 132.0, 132.1, 134.4, 137.4, 141.4, 142.3, 142.7, 147.7, 148.9, 149.8, 151.0, 151.1, 157.4, 157.9, 158.6, 160.4, 161.3, 162.0, 163.2, 163.7, 176.3, 188.3, 189.1. LCMS (ES+) m/z = 1128.0 ([M + H]+, tR = 1.87 min). HRMS (ESI+) m/z = 1127.4014 [M + H]+ found, C62H59O15N6+ required 1127.4033.
(Z)-3-((1-(2-(4-((6-Methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-(4-(2-(4-(((4-oxo-2-phenyl-4H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-4H-chromen-4-one (139). A mixture of alkyne biflavonoid 134 (134 mg, 0.210 mmol), azide aurone 89 (83.5 mg, 0.248 mmol), CuSO4·5H2O (70.8 mg, 0.284 mmol) and sodium ascorbate (131 mg, 0.659 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) to afford triazole hybrid 139 (73.9 mg, 36%) as a bright yellow powdery solid. m.p. 98–100 °C. TLC Rf = 0.39 (7% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 3001w (C-H str), 2929w (C-H str), 2874w (C-H str), 1693m (C=O str), 1632m, 1600s (C=C str), 1509m (C=C str), 1466m, 1444w, 1395m, 1343w, 1248s, 1181s, 1146m, 1129m, 1110m, 1096w, 1044m. 1H-NMR (500 MHz, CDCl3): δ 3.93 (3H, s, -OCH3), 4.34–4.37 (4H, m, 2 × -OCH2CH2N-), 4.69–4.72 (4H, m, 2 × -OCH2CH2N-), 5.32 (4H, s, 2 × -OCH2CN-), 6.77 (1H, d, J = 5.6 Hz, ArH), 6.77 (1H, s, -C=CH), 6.88 (2H, d, J = 8.8 Hz, ArH), 6.90 (2H, d, J = 8.8 Hz, ArH), 7.40–7.45 (6H, m, ArH), 7.51 (1H, d, J = 3.6 Hz, ArH), 7.53 (1H, d, J = 3.6 Hz, ArH), 7.67–7.72 (3H, m, ArH), 7.81 (2H, d, J = 9.2 Hz, ArH), 7.84 (1H, s, -CHN-), 7.88 (1H, s, -CHN-), 8.01–8.05 (4H, m, ArH), 8.27 (2H, dd, J = 8.0, 1.6 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.4, 49.5, 56.0, 64.9, 65.2, 66.2, 66.3, 96.6, 111.5, 112.1, 114.3, 114.9, 115.0, 118.0, 118.1, 124.0, 124.1, 124.1, 124.8, 124.8, 124.9, 125.0, 125.7, 125.7, 125.7, 126.1, 128.4, 128.7, 130.6, 130.7, 133.1, 133.4, 133.6, 139.0, 139.6, 144.0, 144.1, 147.0, 155.2, 155.3, 156.0, 156.4, 158.8, 159.5, 167.3, 168.3, 175.0, 175.1, 182.8. LCMS (ES+) m/z = 975.4 ([M + H]+, tR = 4.94 min). HRMS (ESI+) m/z = 975.2956 [M + H]+ found, C56H43O11N6+ required 975.2984.
2-(4-(2-(4-((4-((E)-3-(Ferrocenyl)acryloyl)-2-methoxyphenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethoxy)phenyl)-3-((1-(2-(4-(((Z)-6-methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-4H-chromen-4-one (140). A mixture of alkyne biflavonoid 135 (71.6 mg, 0.0940 mmol), azide aurone 89 (38.1 mg, 0.113 mmol), CuSO4·5H2O (46.1 mg, 0.185 mmol) and sodium ascorbate (58.0 mg, 0.293 mmol) in t-BuOH/H2O (1:1, 40 mL) was reacted according to GP-A. The crude residue was purified by flash column chromatography (SiO2, 1%–5% MeOH/CH2Cl2) and further purified by preparative HPLC to afford triazole hybrid 140 (2.80 mg, 3%) as a bright red powdery solid. m.p. 158–160 °C. TLC Rf = 0.35 (7% MeOH/CH2Cl2). IR νmax (neat)/cm−1: 2917w (C-H str), 2852w (C-H str), 1737w, 1605s (C=O str), 1509m (C=C str), 1465m, 1443w, 1378w, 1353w, 1260s, 1198m, 1151m, 1133w, 1113w, 1042w, 1015w. 1H-NMR (500 MHz, CDCl3): δ 3.94 (3H, s, -OCH3), 3.96 (3H, s, -OCH3), 4.17 (5H, br s, -C5H5), 4.38 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.42 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.47 (2H, br s, -C5H4), 4.57 (2H, br s, -C5H4), 4.73 (2H, t, J = 5.0 Hz, -OCH2CH2N-), 4.80 (2H, t, J = 4.5 Hz, -OCH2CH2N-), 5.30 (2H, s, -OCH2CN-), 5.41 (2H, s, -OCH2CN-), 6.76–6.79 (2H, m, ArH and -C=CH), 6.77 (1H, d, J = 14.5 Hz, -CH=CHCO-), 6.89 (2H, d, J = 9.0 Hz, ArH), 6.90 (2H, d, J = 8.5 Hz, ArH), 7.14 (1H, d, J = 8.0 Hz, ArH), 7.43 (1H, t, J = 8.0 Hz, ArH), 7.53 (1H, d, J = 8.5 Hz, ArH), 7.58 (1H, d, J = 8.2 Hz, ArH), 7.62 (1H, s, ArH), 7.70 (1H, t, J = 8.5 Hz, ArH), 7.70 (1H, d, J = 16.0 Hz, -CH=CHCO-), 7.71 (2H, d, J = 8.5 Hz, ArH), 7.84 (2H, d, J = 9.0 Hz, ArH), 7.89 (1H, s, -CHN-), 7.94 (1H, s, -CHN-), 8.05 (2H, d, J = 9.0 Hz, ArH), 8.27 (1H, dd, J = 8.0, 1.5 Hz, ArH). 13C-NMR (500 MHz, CDCl3): δ 49.6, 49.7, 56.1, 61.2, 62.8, 66.2, 66.3, 68.9, 69.8, 71.3, 79.3, 96.6, 111.2, 111.6, 111.8, 112.1, 112.3, 114.3, 114.3, 114.9, 118.0, 118.6, 121.3, 122.5, 122.5, 124.1, 124.1, 124.3, 124.4, 124.8, 125.1, 125.7, 125.7, 125.8, 130.6, 133.0, 133.2, 133.5, 138.3, 144.0, 146.0, 149.6, 151.4, 155.1, 157.4, 158.8, 159.5, 167.5, 167.7, 179.2, 182.9, 187.9. LCMS (ES+) m/z = 1098.8 ([M + H]+, tR = 2.02 min). HRMS (ESI+) m/z = 1099.2960 [M + H]+ found, C61H51FeN6O11+ required 1099.2921.