The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides
Abstract
:1. Introduction
2. Results and Discussion
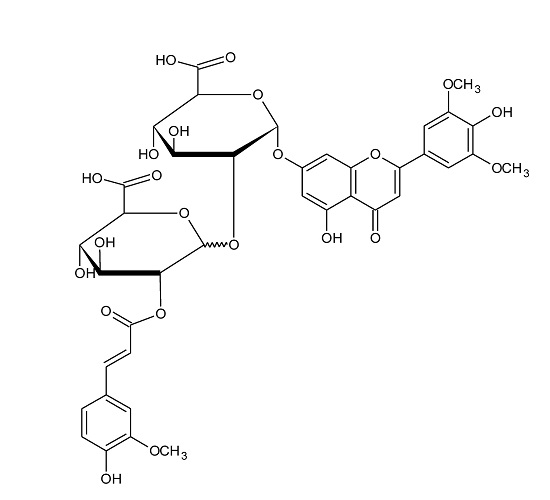
2.1. Flavonoid Glycosides of Axyris amaranthoides
2.2. Fragmentation Pathways of Flavone Glycoconjugates
2.3. Fragmentation Pathways of Acylated Flavone Glycoconjugates
3. Experimental Section
3.1. General
3.2. Plant Materials
3.3. Extraction of Phenolic Compounds
3.4. Liquid Chromatography with Mass Spectrometric Detection
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smolarz, H.D.; Budzianowski, J.; Bogucka-Kocka, A.; Kocki, J.; Mendyk, E. Flavonoid glucuronides with anti-leukaemic activity from Polygonum amphibium L. Phytochem. Anal. 2008, 19, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Poe, M.L.; Bates, A.; Onyilagha, J. Distribution of Leaf Flavonoid Aglycones and Glucuronides in the Genus Phaseolus and Related Genera. Int. J. Biol. 2013, 5, 36–43. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Andersen, O.M.; Markham, K.R. Flavonoids. Chemistry, Biochemistry and Applications; CRC, Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Nazaruk, J.; Gudej, J. Flavonoid compounds from the flowers of Cirsium rivulare (Jacq.) All. Acta Pol. Pharm. Drug Res. 2003, 1, 87–89. [Google Scholar]
- Zhang, Z.; Liu, Y.; Luo, P.; Zhang, H. Separation and Purification of Two Flavone Glucuronides from Erigeron multiradiatus (Lindl.) Benth with Macroporous Resins. J. Biomed. Biotechnol. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Robin, J.; Hoult, S. The flavonoids of Tanacetum parthenium and T-vulgare and their anti-inflammatory properties. Phytochemistry 1999, 51, 417–423. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Zyzyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Tomimori, T.; Miyaichi, Y.; Imoto, Y.; Kizu, H.; Suzuki, C. Studies on the constituents of Scutellaria species. IV. On the flavonoid constituents of the root of Scutellaria baicalensis Georgi (4). Yakugaku Zasshi 1984, 104, 529–534. [Google Scholar] [PubMed]
- Liu, G.; Rajesh, N.; Wang, X.; Zhang, M.; Wu, Q.; Li, S.; Chen, B.; Yao, S. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J. Chromatogr. B 2011, 879, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeap Foo, L. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; De Bruyne, T.; Apers, S.; Ma, Y.; Claeys, M.; Pieters, L.; Vlietinck, A. Flavonoid glucuronides from Picria fel-terrae. Phytochemistry 1999, 52, 1701–1703. [Google Scholar] [CrossRef]
- Yamazaki, K.; Iwashina, T.; Kitajima, J.; Gamou, Y.; Yoshida, A.; Tannowa, T. External and internal flavonoids from Madagascarian Uncarina species (Pedaliaceae). Biochem. Syst. Ecol. 2007, 35, 743–749. [Google Scholar] [CrossRef]
- Aritomi, M.; Kawasaki, T. Three highly oxygenated flavone glucuronides in leaves of Spinacia oleracea. Phytochemistry 1984, 23, 2043–2047. [Google Scholar] [CrossRef]
- Kowalska, I.; Stochmal, A.; Kapusta, I.; Janda, B.; Pizza, C.; Piacente, S.; Oleszek, W. Flavonoids from barrel medic (Medicago truncatula) aerial parts. J. Agric. Food Chem. 2007, 55, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Piacente, S.; Pizza, C.; De Riccardis, F.; Leitz, R.; Oleszek, W. Alfalfa (Medicago sativa L.) Flavonoids. 1. Apigenin and luteolin glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Simonet, A.M.; Macias, F.A.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 2. Tricin and chrysoeriol glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- Marczak, L.; Stobiecki, M.; Jasinski, M.; Oleszek, W.; Kachlicki, P. Fragmentation pathways of acylated flavonoid diglucuronides from leaves of Medicago truncatula. Phytochem. Anal. 2010, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kameda, K.; Kondot, T. Diglucuronoflavones from purple leaves of Perilla ocimoides. Phytochemistry 1993, 33, 917–919. [Google Scholar] [CrossRef]
- Schulz, M.; Strack, D.; Weissenböck, G.; Markham, K.R.; Dellamonica, G.; Chopin, J. Two luteolin O-glucuronides from primary leaves of Secale cereale. Phytochemistry 1985, 24, 343–345. [Google Scholar] [CrossRef]
- Mues, R. Species Specific Flavone Glucuronides in Elodea Species. Biochem. Syst. Ecol. 1983, 11, 261–265. [Google Scholar] [CrossRef]
- Pawlak, K.; Bylka, W.; Jazurek, B.; Matlawska, I.; Sikorska, M.; Manikowski, H.; Bialek-Bylka, G. Antioxidant activity of flavonoids of different polarity, assayed by modified ABTS cation radical decolorization and EPR technique. Acta Biol. Crac. Ser. Bot. 2010, 52, 97–104. [Google Scholar] [CrossRef]
- Budzianowski, J.; Korzeniowska, K.; Chmara, E.; Mrozikiewicz, A. Microvascular protective activity of flavonoid glucuronides fraction from Tulipa gesneriana. Phytother. Res. 1999, 13, 166–168. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China. Vol. 5 (Ulmaceae through Basellaceae); Science Press: Beijing, China, 2003; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003.
- Aswal, B.S.; Bhakuni, D.S.; Goel, A.K.; Kar, K.; Mehrotra, B.N. Screening of Indian plants for biological activity—Part XI. Indian J. Exp. Biol. 1984, 22, 487–504. [Google Scholar] [PubMed]
- Stobiecki, M. Applications of separation techniques hyphenated to mass spectrometer for metabolic profiling. Curr. Org. Chem. 2001, 5, 335–349. [Google Scholar] [CrossRef]
- March, R.E.; Miao, X.S.; Metcalfe, C.D.; Stobiecki, M.; Marczak, L. A fragmentation study of an isoflavone glycoside, genistein-7-O-glucoside, using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 232, 171–183. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, J.; Ran, L.; Lu, C.; Wei, C.; Wang, Y. Analysis of Flavonoids and Hydroxycinnamic Acid Derivatives in Rapeseeds (Brassica napus L. var. napus) by HPLC-PDA-ESI(−)-MSn/HRMS. J. Agric. Food Chem. 2014, 62, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. Practical guidelines for characterization of O-diglycosyl flavonoid isomers by triple quadrupole MS and their applications for identification of some fruit juices flavonoids. J. Mass Spectrom. 2009, 44, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Pikulski, M.; Brodbelt, J.S. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-W.; Hu, W.; Wang, Y.-L.; Huang, L.-F.; He, Y.-B.; Xie, X.-Z. Identification and Determination of Flavonoids in Astragali Radix by High Performance Liquid Chromatography Coupled with DAD and ESI-MS Detection. Molecules 2011, 16, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhu, Z.; Zuo, L.; Chen, L.; Yuan, Q.; Shan, G.; Luo, S.-Z. A strategy for rapid structural characterization of saponins and flavonoids from the testa of Camellia oleifera Abel seeds by ultra-high-pressure liquid chromatography combined with electrospray ionization linear ion trap-orbitrap mass spectrometry. Anal. Methods 2015, 7, 5942–5953. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Leng, J.; Chen, P.; Fan, X.; Cheng, Y. Fragment ion diagnostic strategies for the comprehensive identification of chemical profile of Gui-Zhi-Tang by integrating high-resolution MS, multiple-stage MS and UV information. J. Pharm. Biomed. Anal. 2014, 98, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Sik, V.; Rees, H.H.; Dinan, L. 1α,20R-dihydroxyecdysone from Axyris amaranthoides. Phytochemistry 1998, 49, 2305–2310. [Google Scholar] [CrossRef]
- Dinan, L. Distribution of phytoecdysteroids within Chenopodiaceae. Eur. J. Entomol. 1995, 92, 295–300. [Google Scholar]
- Wojakowska, A.; Piasecka, A.; García-López, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC-MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A.; Aráiz-Hernández, D.; Alvarez, M.M.; Serna-Saldivar, S.O. Induction of Apoptosis in Colon Cancer Cells Treated with Isorhamnetin Glycosides from Opuntia Ficus-indica Pads. Plant Foods Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Matlawska, I. Kaempferol, isorhamnetin and their glycosides in the flowers of Asclepias syriaca L. Acta Pol. Pharm. Drug Res. 2001, 58, 269–272. [Google Scholar]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural characterization of flavonoid glycosides by multi-stage mass spectrometry. Mass Spectrom. Rev. 2008, 29. [Google Scholar] [CrossRef] [PubMed]
- Kachlicki, P.; Einhorn, J.; Muth, D.; Kerhoas, L.; Stobiecki, M. Evaluation of glycosylation and malonylation patterns in flavonoid glycosides during LC/MS/MS metabolite profiling. J. Mass Spectrom. 2008, 43, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, S.W. Quantitative changes of polyphenolic compounds in mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev. Nutr. Food Sci. 2012, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Hartley, R.C.; Crozier, A. Detection and identification of 14C-labelled flavonol metabolites by high-performance liquid chromatography-radiocounting and tandem mass spectrometry. J. Chromatogr. A 2003, 1007, 21–29. [Google Scholar] [CrossRef]
- Dobberstein, D.; Bunzel, M. Identification of ferulate oligomers from corn stover. J. Sci. Food Agric. 2010, 90, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Renger, A.; Steinhart, H. Ferulic acid dehydrodimers as structural elements in cereal dietary fibre. Eur. Food Res. Technol. 2000, 211, 422–428. [Google Scholar] [CrossRef]
- Ford, W.C.; Hartley, D.R. GC/MS characterization of cyclodimers from p-coumaric and ferulic acids by photodimerization—A possible factor influencing cell wall biodegradability. J. Sci. Food Agric. 1989, 46, 301–310. [Google Scholar] [CrossRef]
- Micard, V.; Grabber, J.H.; Ralph, J.; Renard, C.M.G.C.; Thibault, J.F. Dehydrodiferulic acids from sugar-beet pulp. Phytochemistry 1997, 44, 1365–1368. [Google Scholar] [CrossRef]
- Stobiecki, M.; Busko, M.; Marczak, L.; Bednarek, P.; Pislewska, M.; Wojtaszek, P. The complexity of oxidative cross-linking of phenylpropanoids: Evidence from an in vitro model system. Funct. Plant Biol. 2002, 29, 853–864. [Google Scholar] [CrossRef]
- Svedström, U.; Vuorela, H.; Kostiainen, R.; Laakso, I.; Hiltunen, R. Fractionation of polyphenols in hawthorn into polymeric procyanidins, phenolic acids and flavonoids prior to high-performance liquid chromatographic analysis. J. Chromatogr. A 2006, 1112, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not availaible.





| # | Name | Elemental Composition | Exact Monoisotopic Mass (Da) | Fragment Ions in Positive Ion Mode (m/z) | Fragment Ions in Negative Ion Mode (m/z) |
|---|---|---|---|---|---|
| 1 | Isorhamnetin 3(7)-O-glucuronopyranosyl-(1-2)-O-glucuronopyranoside | C28H28O19 | 668.1224 | 669, 493, 317 | 667, 351, 315, 193, 175 |
| 2 | Chrysoeriol 7-O-glucuronopyranosyl-(1-2)-O-glucuronopyranoside | C28H28O18 | 652.1275 | 653, 477, 301 | 651, 351, 299, 175 |
| 3 | Chrysoeriol 7-O-[2-O-feruloyl-glucuronopyranosyl-(1-3)-O-glucopyranoside] | C38H38O20 | 814.1956 | 815, 653, 477 | 813, 513 |
| 4 | Luteolin 7-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C37H34O21 | 814.1592 | 815, 463,2 87 | 814, 527, 285, 193 |
| 5 | Tricin 7-O-glucuronopyranosyl-(1-2)-O-glucuronopyranoside | C29H30O19 | 682.1381 | 683, 507, 331 | 681, 351, 193, 175 |
| 6 | Tricin 7-O-[2-O-feruloyl-glucopyranosyl-(1-3)-O-glucuronopyranoside] | C39H40O21 | 844.2062 | 845, 683, 507 | 843, 513 |
| 7 | Tricin 7-O-{2′-O-feruloyl-[glucopyranosyl-(1-3′)-O-glucopyranosyl]-(1-2)-O-glucuronopyranoside} | C45H50O26 | 1006.259 | 1007, 507 | 1005, 675, 513 |
| 8 | Isorhamnetin 3(7)-O-glucuronopyranoside | C22H20O13 | 492.0904 | 493, 317 | 491, 315 |
| 9 | Isorhamnetin 3(7)-O-{3′-O-coumaroyl-[glucuronopyranosyl-(1-2′)-O-glucopyranosyl]-(1-2)-O-glucuronopyranoside} | C43H44O26 | 976.212 | 977, 815, 493, 317 | 975, 811, 659, 513, 495, 337, 315 |
| 10 | Syringetin 3-O-[2′-O-feuroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C39H38O23 | 874.1804 | 875, 523, 347 | 873, 527, 333, 193 |
| 11 | Apigenin 7-O-{2′-O-feruloyl-[glucopyranosyl-(1-3′)]-O-glucuronopyranosyl-(1-2)-O-glucuronopyranoside} | C43H44O25 | 960.2171 | 961, 799, 447, 271 | 959, 689, 527, 193 |
| 12 | Isorhamnetin 3(7)-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C38H36O22 | 844.1698 | 845, 493, 317, 177 | 843, 649, 527, 315, 193 |
| 13 | Chrysoeriol 7-O-{3′-O-feruloyl-[glucuronopyranosyl-(1-2′)]-O-glucopyranosyl-(1-2)-O-glucuronopyranoside} | C44H46O26 | 990.2277 | 991, 829, 477, 301 | 989, 689, 495 |
| 14 | Tricin 7-O-{3′-O-feruloyl-[glucuronopyranosyl-(1-2)]-O-glucopyranosyl-(1-2)-O-glucuronopyranoside} | C45H48O27 | 1020.238 | 1021, 859, 507, 331 | 1019, 689, 495 |
| 15 | Tricin 7-O-{3′-O-coumaroyl-[glucuronopyranosyl-(1-2′)]-O-glucopyranosyl-(1-2)-O-glucuronopyranoside} | C44H46O26 | 990.2277 | 991, 829, 507, 331 | 989, 659, 495 |
| 16 | Tricin 7-O-glucuronopyranoside | C23H22O13 | 506.105 | 507, 331 | 505, 490, 329, 314 |
| 17 | Tricin 7-O-[2′-O-sinapoyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C40H40O23 | 888.196 | 889, 507, 383, 331, 207 | 887, 557, 399, 223 |
| 18 | Chrysoeriol 7-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C38H36O21 | 828.1749 | 829, 477, 353, 301, 177 | 827, 527, 333, 193 |
| 19 | Tricin 7-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C39H38O22 | 858.1854 | 859, 507, 331 | 857, 527, 193 |
| 20 | Tricin 7-O-[2′-O-coumaroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C38H36O21 | 828.1749 | 829, 507, 331, 147 | 827, 497, 333, 163 |
| 21 | Tricin 7-O-{3-O-feruloyl-[glucopyranosyl-(1-2′)-O-glucuronopyranosyl]-(1-2)-O-glucuronopyranoside} | C45H48O27 | 1020.238 | 1021, 683, 507, 339, 177 | 1019, 689, 495 |
| 22 | Tricin 5-O-glucuronopyranosyl-7-O-glucopyranoside | C29H32O18 | 668.1588 | 669, 507, 331 | 667, 491, 329 |
| 23 | Tricin 7-O-[2′-O-5-hydroxyferuloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C39H38O23 | 874.1803 | 875, 507, 369, 331 | 873, 543, 351, 209 |
| 24 | Chrysoeriol 4′-O-xylopyranosyl-7-O-[2′-O-coumaroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C42H42O24 | 930.2066 | 931, 799, 609, 433, 301 | 929, 797, 497, 163 |
| 25 | Tricin 4′-O-xylopyranosyl-7-O-[2′-O-coumaroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C43H44O25 | 960.2171 | 961, 829, 507, 463, 331 | 959, 827, 497 |
| 26 | Tricin 7-O-[glucuronopyranosyl-(1-2)-O-methyloglucuronopyranoside] | C30H32O19 | 696.1537 | 697, 521, 331 | 695, 533, 329 |
| 27 | Isorhamnetin 3(7)-O-[2′-O-coumaroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C37H34O21 | 814.1592 | 815, 493, 317 | 813, 649, 497, 315, 163 |
| 28 | Chrysoeriol 7-O-glucuronopyranoside | C22H20O12 | 476.0954 | 477, 301 | - |
| 29 | Apigenin 7-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C37H34O20 | 798.1643 | 799, 447, 331, 271 | 797, 603, 527, 333, 193 |
| 30 | Chrysoeriol 7-O-[2′-O-coumaroyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C37H34O20 | 798.1643 | 799, 477, 301 | 797, 497 |
| 31 | Tricin 7-O-[2′-O-feruloyl-glucuronopyranosyl-(1-2)-O-methyloglucuronopyranoside] | C40H40O22 | 872.2011 | 873, 521, 331, 177 | 871, 677, 519, 329 |
| 32 | Tricin 7-O-[2′-O-benzoyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C36H34O20 | 786.1643 | 787, 507, 331 | 785, 455, 333, 121 |
| 33 | Tricin 7-O-[2′-O-coumaroyl-methyloglucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C39H38O21 | 842.1905 | 843, 507, 331 | 841, 695, 633, 511, 329, 163 |
| 34 | Tricin 7-O-[2′-O-dehydrodiferuloyl-glucopyranosyl-(1-2)-O-glucuronopyranoside] | C49H48O25 | 1036.2479 | 1037, 531, 331 | 1035, 705, 547, 371 |
| 35 | Tricin 7-O-[2′-O-dehydrodiferuloyl-glucuronopyranosyl-(1-2)-O-glucuronopyranoside] | C49H48O26 | 1052.2428 | 1053, 859, 507, 331 | 1051, 721, 563, 387 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marczak, Ł.; Znajdek-Awiżeń, P.; Bylka, W. The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides. Molecules 2016, 21, 1229. https://doi.org/10.3390/molecules21091229
Marczak Ł, Znajdek-Awiżeń P, Bylka W. The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides. Molecules. 2016; 21(9):1229. https://doi.org/10.3390/molecules21091229
Chicago/Turabian StyleMarczak, Łukasz, Paulina Znajdek-Awiżeń, and Wiesława Bylka. 2016. "The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides" Molecules 21, no. 9: 1229. https://doi.org/10.3390/molecules21091229