Struvite Precipitation as a Means of Recovering Nutrients and Mitigating Ammonia Toxicity in a Two-Stage Anaerobic Digester Treating Protein-Rich Feedstocks
Abstract
:1. Introduction
2. Results and Discussions
2.1. Selection of Mg and P Sources for SP Test
2.2. Experiment 1—Optimization of Struvite Precipitation
2.3. Experiment 2—Biochemical Methane Potential of TAN Reduced Substrates
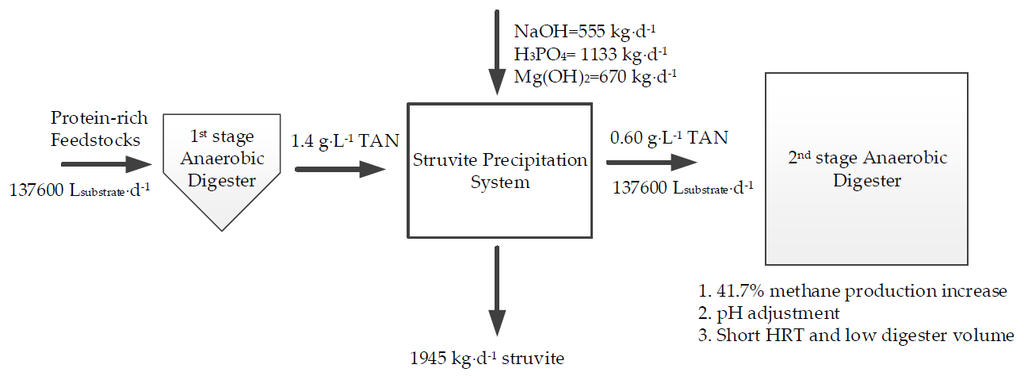
2.4. Optimizatiom of SP Treatment
2.5. Economic Analysis
3. Materials and Methods
3.1. Substrates
3.2. Experiment 1-Optimization of Struvite Precipitation
3.2.1. Reagents
3.2.2. Struvite Precipitation Protocol
3.3. Experiment 2- Biochemical Methane Potential of TAN Reduced Substrates
3.3.1. Treatments Used in Experiments
3.3.2. Inoculum
3.3.3. Biochemical Methane Potential (BMP) Assay
3.3.4. Calculation of Cumulative Methane Production
3.3.5. Modeling the Kinetics of Methane Production
3.3.6. Analytical Methods
3.3.7. Statistical and Regression Method
3.3.8. Economic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Salminen, E.; Rintala, J. Anaerobic digestion of organic solid poultry slaughterhouse waste—A review. Bioresour. Technol. 2002, 83, 13–26. [Google Scholar] [CrossRef]
- Bouallagui, H.; Haouari, O.; Touhami, Y.; Ben Cheikh, R.; Marouani, L.; Hamdi, M. Effect of temperature on the performance of an anaerobic tubular reactor treating fruit and vegetable waste. Process Biochem. 2004, 39, 2143–2148. [Google Scholar] [CrossRef]
- Edström, M.; Nordberg, A.; Thyselius, L. Anaerobic treatment of animal byproducts from slaughterhouses at laboratory and pilot scale. Appl. Biochem. Biotechnol. 2003, 109, 127–138. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Oh, S.; Kim, C. Potential of anaerobic digestion for material recovery and energy production in waste biomass form a poultry slaughterhouse. Waste Manag. 2014, 34, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Banks, C.J. Evaluation of a two stage anaerobic digester for the treatment of mixed abattoir wastes. Process Chem. 2003, 38, 1267–1273. [Google Scholar] [CrossRef]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Choi, C.U.; Lee, M.J.; Chung, I.H.; Kim, D.S. A study of NH3-N and P refixation by struvite formation in hybrid anaerobic reactor. Water Sci. Technol. 2004, 49, 207–214. [Google Scholar] [PubMed]
- Celen, I.; Türker, M. Recovery of ammonia from anaerobic digester effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P and determination of rate constant. Bioresour. Technol. 2003, 89, 229–136. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Astals, S.; Chimenos, J.M.; Martinez, M.; Mata-Alvarez, J. Improving anaerobic digestion of pig manure by adding in the same reactor a stabilizing agent formulated with low-grade magnesium oxide. Biomass Bioenergy 2014, 67, 243–251. [Google Scholar] [CrossRef]
- Chemister. Available online: chemister.ru/Database/search-en.php (accessed on 15 February 2016).
- FAO. Available online: http://www.fao.org/ag/agn/jecfa-additives/specs/Monograph1/Additive-261.pdf (accessed on 15 February 2016).
- Gerardi, M.H. The Microbiology of Anaerobic Digesters, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; pp. 105–115. [Google Scholar]
- Hamilton, D.W. Anaerobic Digestion of Animal Manures: Inhibitory and Toxic Materials; BAE-1763; Oklahoma Cooperative Extension Service: Oklahoma State University, Stillwater, OK, USA, 2013. [Google Scholar]
- Vijayaraghavan, K.; Ramanujam, T.K. Effect of chloride and condensable tannin in anaerobic degradation of tannery wastewaters. Bioprocess Eng. 1999, 20, 499–503. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Demirer, G.N.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, C.; Li, P.; Jiang, R. Application of struvite process for nutrient recovery from anaerobic digesters of livestock wastewater. Environ. Prot. Eng. 2014, 40, 29–42. [Google Scholar]
- Yetilmezsoy, K.; Sapci-Zengin, Z. Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J. Hazard. Mater. 2009, 166, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, X.; Zuo, J.; Liu, Y.; Duan, E.; Yang, J.; Chen, P.; Wang, Y. Struvite precipitation for ammonia nitrogen removal in 7-Aminocephalosporanic acid wastewater. Molecules 2012, 17, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Altinbas, M.; Ozturk, I.; Aydin, A.F. Ammonia recovery from high strength agro industry effluents. Water Sci. Technol. 2002, 45, 189–196. [Google Scholar] [PubMed]
- Altinbas, M.; Yangin, C.; Ozturk, I. Struvite precipitation from anaerobically treated municipal and landfill wastewaters. Water Sci. Technol. 2002, 46, 271–278. [Google Scholar] [PubMed]
- Sugiyama, S.; Yokoyama, M.; Ishizuka, H.; Sotowa, K.; Tomida, T.; Shigemoto, N. Removal of aqueous ammonium with magnesium phosphates obtained from the ammonium-elimination of magnesium ammonium phosphate. J. Colloid Interface Sci. 2005, 292, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Türker, M.; Celen, I. Chemical equilibrium model of struvite precipitation from anaerobic digester effluents. Turk. J. Eng. Environ. Sci. 2010, 34, 39–48. [Google Scholar]
- Cuetos, M.J.; Moran, A.; Otero, M.; Gomez, X. Anaerobic co-digestion of poultry blood with OFMSW: FTIR and TG-DTG study of process stabilization. Environ. Technol. 2009, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Schattauer, A.; Abdoun, E.; Weiland, P.; Plochl, M.; Heiermann, M. Abundance of trace elements in demonstration biogas plants. Biosyst. Eng. 2011, 108, 57–65. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The thermal decomposition of huntite and hydromagnesite—A review. Thermochim. Acta 2010, 509, 1–11. [Google Scholar] [CrossRef]
- Banach, M.; Makara, A. Thermal decomposition of sodium phosphates. J. Chem. Eng. Data 2011, 56, 3095–3099. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Stratful, I.; Scrimshaw, M.D.; Lester, J.N. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic waste treatment fundamentals. Public Works 1964, 95, 107–112. [Google Scholar]
- Wang, R.; Li, Y.; Wang, W.; Chen, Y.; Vanrolleghem, P.A. Effect of high orthophosphate concentration on mesophilic anaerobic sludge digestion and its modeling. Chem. Eng. J. 2015, 260, 791–800. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, J.; Zhang, Z.; Sugiura, N. Methane production from rice straw with acclimated anaerobic sludge: effect of phosphate supplementation. Bioresour. Technol. 2010, 101, 4343–4348. [Google Scholar] [CrossRef] [PubMed]
- Münch, E.V.; Barr, K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001, 35, 151–159. [Google Scholar] [CrossRef]
- Birnhack, L.; Nir, O.; Telzhenski, M.; Lahav, O. A new algorithm for design, operation and cost assessment of struvite (MgNH4PO4) precipitation processes. Environ. Technol. 2015, 36, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Tecnon OrbiChem. Available online: http://www.orbichem.com/userfiles/CNF%20Samples/cas_13_11.pdf (accessed on 15 February 2016).
- ICIS. Available online: http://www.icis.com/chemicals/channel-info-chemicals-a-z/ (accessed on 15 February 2016).
- Kramer, D.A. Magnesium compounds. In USGS Minerals Yearbook: Volume I, Metals and Minerals; U.S. Geological Survey: Washington DC, USA, 2007; pp. 461–469. [Google Scholar]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Available online: http://www.eia.gov/dnav/ng/ng_pri_sum_dcu_nus_a.htm (accessed on 26 April 2016).
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, S.; Shin, K.; Kim, C. Effects of substrate to inoculums ratio on the biochemical methane potential of piggery slaughterhouse wastes. Asian Australas. J. Anim. Sci. 2014, 27, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Pollution Control Federation: Washington DC, USA, 1992.
- Kiepper, B.H. Tertiary Microscreening of Poultry Processing Wastewater. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2007. [Google Scholar]
- Sample Availability: Not available.



| Molar Weight 1 (g·mol−1) | Solubility 1 (g per 100 g Water, 25 °C) | Toxic Residual 2 | Final Concentration 3 (g·L−1) | Inhibition Threshold 4 (g·L−1) | |
|---|---|---|---|---|---|
| Mg sources | |||||
| MgCl2 | 95.2 | 55.5 | Cl | 7.1 | 5.5 |
| MgO | 40.3 | 0.0086 (30 °C) | None | None | None |
| Mg(OH)2 | 58.3 | 0.00064 | None | None | None |
| MgCO3 | 84.3 | 0.0139 | None | None | None |
| MgSO4 | 120.4 | 37.4 | S | 3.2 | 0.05 |
| Mg(NO3)2 | 148.3 | 72.7 | N | 2.8 | 1.5 |
| MgHPO4 | 120.3 | Slightly soluble | None | None | None |
| P sources | |||||
| H3PO4 | 98.0 | 599.3 (24 °C) | None | None | None |
| NaH2PO4 | 120.0 | 85.2 (20 °C) | Na | 2.3 | 3.5–5.5 |
| Na2HPO4 | 142.0 | 12.0 | Na | 4.6 | |
| Na3PO4 | 163.9 | 14.5 | Na | 6.9 | |
| KH2PO4 | 136.1 | 25.1 | K | 3.9 | 2.5–4.5 |
| K2HPO4 | 174.2 | 168.4 | K | 7.8 | |
| K3PO4 | 212.3 | 105.9 | K | 11.7 | |
| Parameters | Avg. ± Std. Dev. | n 1 |
|---|---|---|
| pH | 6.4 ± 0.1 | 62 |
| TS (g·L−1) | 2.7 ± 0.2 | 23 |
| VS (g·L−1) | 1.7 ± 0.1 | 23 |
| TSS (g·L−1) | 1.2 ± 0.2 | 23 |
| COD (g·L−1) | 13.3 ± 0.7 | 25 |
| TAN (g·L−1) | 1.3 ± 0.1 | 25 |
| VFA (g acetic acid L−1) | 10.6 ± 1.8 | 27 |
| Group | TAN at T0 1 (g·L−1) | TAN at T20 1 (g·L−1) | TAN Removal (%) | NaOH Used (g·L−1substrate) | pH0 2 | pH20 3 | T20 3 (°C) | ||
|---|---|---|---|---|---|---|---|---|---|
| Rep 1 | Rep 2 | AVG | |||||||
| G1 | 1.386 | 0.345 | 0.277 | 0.311 | 77.5 | 4.8 | 5.9 | 8.5 | 26.7 |
| G2 | 1.336 | 0.714 | 0.609 | 0.661 | 50.5 | 2.6 | 6.4 | 8.5 | 25.5 |
| G3 | 1.409 | 0.159 | 0.150 | 0.155 | 89.0 | 12.5 | 3.2 | 8.4 | 25.0 |
| G4 | 1.514 | 0.177 | 0.177 | 0.177 | 88.3 | 8.3 | 5.4 | 8.7 | 23.8 |
| G5 | 1.386 | 0.377 | 0.341 | 0.359 | 74.1 | 4.8 | 5.8 | 8.5 | 24.2 |
| G6 | 1.364 | 0.632 | 0.491 | 0.561 | 58.8 | 2.0 | 6.5 | 8.9 | 22.5 |
| G7 | 1.455 | 0.364 | 0.336 | 0.350 | 75.9 | 7.5 | 5.8 | 8.5 | 22.1 |
| G8 | 1.459 | 0.318 | 0.368 | 0.343 | 76.5 | 3.6 | 6.3 | 8.4 | 21.4 |
| G9 | 1.473 | 1.386 | 1.405 | 1.395 | 5.2 | 0.4 | 6.6 | 8.2 | 20.8 |
| Parameters 1 | G0 4 | G1 | G3 | G5 | G7 | G8 |
|---|---|---|---|---|---|---|
| pH | 7.1 | 9.0 | 8.7 | 9.2 | 8.9 | 8.9 |
| TS (g·L−1) | 2.9 ± 0.2 | 14.3 ± 0.1 | 21.8 ± 0.2 | 13.9 ± 0.1 | 16.5 ± 0.2 | 17.1 ± 0.2 |
| VS (g·L−1) | 1.8 ± 0.2 | 5.9 ± 0.2 | 4.9 ± 0.1 | 5.9 ± 0.0 | 5.6 ± 0.1 | 5.9 ± 0.1 |
| COD (g·L−1) | 14.7 ± 3.3 | 13.3 ± 1.2 | 12.5 ± 2.0 | 12.2 ± 0.7 | 11.4 ± 0.1 | 12.6 ± 1.6 |
| TN (g·L−1) | 1.5 ± 0.0 | 0.7 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| TAN (g·L−1) | 1.4 ± 0.0 | 0.6 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| C/N 2 | 5.0 | 14.8 | 22.7 | 17.0 | 22.1 | 21.8 |
| M:A:P 3 | 1:310:8 | 1:11:11 | 1:6:6 | 1:18:18 | 1:19:19 | 1:24:23 |
| Micronutrients (ppm or mg·L−1) | ||||||
| Al | 1.79 | 1.53 | 8.81 | <0.50 | 4.42 | 0.86 |
| B | <0.20 | <0.20 | <0.20 | <0.20 | 0.46 | 0.43 |
| Ca | 27.88 | 33.10 | 21.22 | 22.96 | 37.22 | 47.74 |
| Cd | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Cr | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Cu | 0.75 | 1.00 | 0.41 | 0.38 | 0.31 | 0.27 |
| Fe | 15.35 | 12.39 | 11.71 | 10.86 | 9.83 | 12.18 |
| K | 123.0 | 121.6 | 96.2 | 118.8 | 124.4 | 117.5 |
| Mg | 7.83 | 90.44 | 26.52 | 42.70 | 28.60 | 23.96 |
| Mn | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Mo | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Na | 269 | 3526 | 7432 | 3618 | 4856 | 4892 |
| Ni | <0.20 | <0.20 | <0.20 | <0.20 | <0.20 | <0.20 |
| P | 79 | 1246 | 195 | 1005 | 706 | 726 |
| Pb | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 | <0.50 |
| S | 54.56 | 56.94 | 49.32 | 52.78 | 55.84 | 56.46 |
| Si | 7.56 | 7.60 | 20.54 | 10.45 | 12.64 | 10.11 |
| Zn | 0.40 | 0.28 | <0.10 | <0.10 | 0.19 | 0.26 |
| Treatments 1 | DI Water (mL) | Inoculum (mL) | Untreated Substrate (mL) | Treated Substrates 2 (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G3 | G5 | G7 | G8 | |||
| BI | 210 | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| BG0 | 0 | 90 | 210 | 0 | 0 | 0 | 0 | 0 |
| BG1 | 0 | 90 | 0 | 210 | 0 | 0 | 0 | 0 |
| BG3 | 0 | 90 | 81 | 0 | 129 | 0 | 0 | 0 |
| BG5 | 0 | 90 | 34 | 0 | 0 | 176 | 0 | 0 |
| BG7 | 0 | 90 | 53 | 0 | 0 | 0 | 157 | 0 |
| BG8 | 0 | 90 | 51 | 0 | 0 | 0 | 0 | 159 |
| Parameters | BG0 | BG1 | BG3 | BG5 | BG7 | BG8 |
|---|---|---|---|---|---|---|
| Pm 1 (mL) | 593.2 ± 213.1 | 367.9 ± 12.0 | 516.8 ± 326.5 | 559.3 ± 22.4 | 430.7 ± 27.9 | 400.8 ± 61.3 |
| Rm 1 (mL·day−1) | 2.9 ± 0.4 | 4.3 ± 0.1 | 1.6 ± 0.6 | 2.6 ± 0.4 | 1.9 ± 0.0 | 2.8 ± 1.4 |
| λ 1 (day) | 80.5 ± 9.7 | 125.7 ± 7.6 | 20.9 ± 29.6 | 8.7 ± 4.3 | 18.3 ± 0.4 | 68.6 ± 27.5 |
| Methane yield 2 (mL·g−1 CODadded) | 127.2 b ± 9.3 | 116.8 b ± 4.6 | 123.3 b ± 33.7 | 180.2 a ± 9.2 | 125.8 b ± 2.1 | 112.6 b ± 4.8 |
| Methane yield 2 (mL·g−1 CODremoved) | 263.7 ± 38.1 | 233.9 ± 7.8 | 267.5 ± 131.7 | 277.5 ± 10.2 | 255.6 ± 7.9 | 270.6 ± 52.6 |
| COD (g·L−1) | TAN (g·L−1) | Na (mg·L−1) | P (mg·L−1) | Cl− (mg·L−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| B | A | Removal 1 (%) | B | A | Removal 1 (%) | B | B | A | |
| BG0 | 10.77 | 5.45 ± 1.25 | 49 ± 12 | 1.42 | 1.26 ± 0.03 | 12 a ± 2 | 336 | 80 | 336 |
| BG1 | 9.79 | 4.90 ± 0.25 | 50 ± 3 | 0.86 | 0.79 ± 0.03 | 7 ab ± 4 | 2616 | 897 | 335 |
| BG3 | 9.82 | 4.69 ± 1.83 | 52 ± 19 | 0.85 | 0.85 ± 0.04 | 1 b ± 4 | 3416 | 130 | 3026 |
| BG5 | 9.30 | 3.26 ± 0.20 | 65 ± 2 | 0.85 | 0.84 ± 0.03 | 1 b ± 3 | 2300 | 623 | 352 |
| BG7 | 9.04 | 4.59 ± 0.21 | 49 ± 2 | 0.85 | 0.84 ± 0.01 | 1 b ± 1 | 2736 | 408 | 364 |
| BG8 | 9.66 | 5.56 ± 0.58 | 42 ± 6 | 0.85 | 0.79 ± 0.03 | 7 ab ± 3 | 2786 | 422 | 360 |
| Unit Price ($·kg−1) | Mass Flow (kg·d−1) | Money Flow ($·d−1) | |
|---|---|---|---|
| Mg source | MgO | ||
| Input | |||
| NaOH | 0.44 1 | 660 | 287.31 |
| H3PO4 | 0.45 2 | 1348 | 606.82 |
| MgO | 0.55 3 | 550 | 302.72 |
| Output | |||
| Struvite (MgNH4PO4·6H2O) | 0.84 4 | 1935 | 1631.26 |
| Methane 5 | 0.38 | 0 | 0 |
| Net benefit ($·d−1) | 434.42 | ||
| Mg source | Mg(OH)2 | ||
| Input | |||
| NaOH | 0.44 1 | 555 | 241.34 |
| H3PO4 | 0.45 2 | 1133 | 509.73 |
| Mg(OH)2 | 0.28/0.99 3 | 670 | 187.71/663.68 |
| Output | |||
| Struvite (MgNH4PO4·6H2O) | 0.84 4 | 1945 | 1640.02 |
| Methane 5 | 0.38 | 97 | 36.86 |
| Net benefit ($·d−1) | 738.10/262.13 | ||
| Group | Mg and P Source | Molar Weight (g·mol−1) | Amount (g) | Ions Left in the Solutions (mg·L−1) | |
|---|---|---|---|---|---|
| Na+ | Cl− | ||||
| G1 | MgO | 40.3 | 1.94 | 0 | 0 |
| H3PO4 (85%) | 98.0 | 5.6 | |||
| G2 | MgO | 40.3 | 1.94 | 2233 | 0 |
| NaH2PO4·H2O | 138.0 | 6.7 | |||
| G3 | MgCl2·6H2O | 203.3 | 9.86 | 0 | 6887 |
| H3PO4 (85%) | 98.0 | 5.6 | |||
| G4 | MgCl2·6H2O | 203.3 | 9.86 | 2233 | 6887 |
| NaH2PO4·H2O | 138.0 | 6.7 | |||
| G5 | Mg(OH)2 | 58.3 | 2.82 | 0 | 0 |
| H3PO4 (85%) | 98.0 | 5.6 | |||
| G6 | Mg(OH)2 | 58.3 | 2.82 | 2233 | 0 |
| NaH2PO4·H2O | 138.0 | 6.7 | |||
| G7 | MgCO3 | 84.3 | 4.08 | 0 | 0 |
| H3PO4 (85%) | 98.0 | 5.6 | |||
| G8 | MgCO3 | 84.3 | 4.08 | 2233 | 0 |
| NaH2PO4·H2O | 138.0 | 6.7 | |||
| G9 | MgHPO4·3H2O | 174.3 | 8.45 | 0 | 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hawkins, G.L.; Kiepper, B.H.; Das, K.C. Struvite Precipitation as a Means of Recovering Nutrients and Mitigating Ammonia Toxicity in a Two-Stage Anaerobic Digester Treating Protein-Rich Feedstocks. Molecules 2016, 21, 1011. https://doi.org/10.3390/molecules21081011
Wang S, Hawkins GL, Kiepper BH, Das KC. Struvite Precipitation as a Means of Recovering Nutrients and Mitigating Ammonia Toxicity in a Two-Stage Anaerobic Digester Treating Protein-Rich Feedstocks. Molecules. 2016; 21(8):1011. https://doi.org/10.3390/molecules21081011
Chicago/Turabian StyleWang, Shunli, Gary L. Hawkins, Brian H. Kiepper, and Keshav C. Das. 2016. "Struvite Precipitation as a Means of Recovering Nutrients and Mitigating Ammonia Toxicity in a Two-Stage Anaerobic Digester Treating Protein-Rich Feedstocks" Molecules 21, no. 8: 1011. https://doi.org/10.3390/molecules21081011