Intra-Population Variation of Secondary Metabolites in Cistus ladanifer L.
Abstract
:1. Introduction
2. Results
2.1. Secretion of Secondary Metabolites
2.2. Spatial Distribution of the Individuals of the Studied Population
3. Discussion
4. Materials and Methods
4.1. Description of C. ladanifer
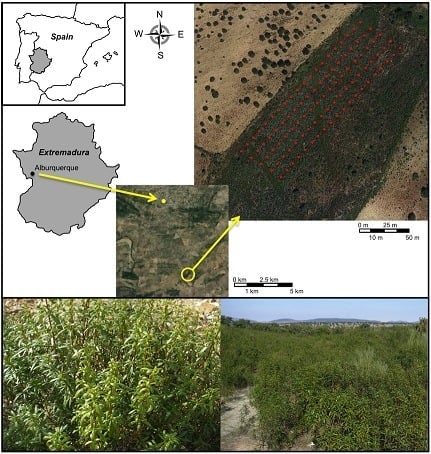
4.2. Study Area and Sample Collection
4.3. Extraction and Assay of Secondary Metabolites
4.4. Statistical Analyses
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suomela, J.; Ossipov, V.; Haukioja, E. Variation among and within mountain birch trees in foliage phenols, carbohydrates and amino acids and in growth of Epirrita autumnata larvae. J. Chem. Ecol. 1995, 21, 1421–1446. [Google Scholar] [CrossRef] [PubMed]
- Holeski, L.M.; Hillstrom, M.L.; Whitham, T.G.; Lindroth, R.L. Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 2012, 170, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Zangerl, A.R.; Berenbaum, M.R. Heritability estimates for octyl-acetate and octyl-butyrate in the mature fruit of the wild parsnip. J. Hered. 2000, 91, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Orians, C.M.; Lower, S.; Fritz, R.S.; Roche, B.M. The effects of plant genetic variation and soil nutrients on secondary chemistry and growth in a shrubby willow, Salís sericea: Patterns and constraints on the evolution of resistance traits. Biochem. Syst. Ecol. 2003, 31, 233–247. [Google Scholar] [CrossRef]
- Lomba, A.; Alves, P.; Honrado, J. Endemic sand dune vegetation of the Northwest Iberian Peninsula: Diversity, dynamics, and significance for bioindication and monitoring of coastal landscapes. J. Coast. Res. 2008, 24, 113–121. [Google Scholar] [CrossRef]
- Herrera, C.M. Multiplicity in Unity: Plant Subindividual Variation and Interactions with Animals; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 2006, 201, 163–177. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Gould, K.S. Anthocyanin function in vegetative organs. In Anthocyanins; Gould, K.S., Davies, K., Winefield, C., Eds.; Springer: New York, NY, USA, 2009; pp. 1–19. [Google Scholar]
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Seasonal variation of exudate of Cistus ladanifer. J. Chem. Ecol. 1993, 19, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Borges, A. Study of Cistus ladanifer L. Its Importances in Portugal; Instituto Nacional de Investigaçao Agrária, Estaçao Forestal Nacional: Lisbon, Portugal, 1988. [Google Scholar]
- Chaves, N.; Ríos, J.L.; Gutiérrez, C.; Escudero, J.C.; Olías, J.M. Analysis of secreted flavonoids of Cistus ladanifer L. by high-performance liquid chromatography-particle beam mass spectrometry. J. Chromatogr. A 1998, 799, 111–115. [Google Scholar] [CrossRef]
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational variation in flavonoid composition of Cistus ladanifer L. exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Alías, J.C. Influencia de los Factores Climáticos en la Síntesis y Actividad de Compuestos Fitotóxicos Secretados por Cistus ladanifer L. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 2006. [Google Scholar]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal variation of Cistus ladanifer L. diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N. Variación Cualitativa y Cuantitativa de los Flavonoides del Exudado de Cistus ladanifer L. Como Respuesta a Diferentes Factores Ecológicos. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 1994. [Google Scholar]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant growth inhibiting flavonoids in exudate of Cistus ladanifer and in associated soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Sosa, T.; Chaves, N.; Alías, J.C.; Escudero, J.C.; Henao, F.; Gutiérrez-Merino, C. Inhibition of mouth skeletal muscle relaxation by flavonoids of Cistus ladanifer L.: A plant defense mechanism against herbivores. J. Chem. Ecol. 2004, 30, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Alías, J.C.; Sosa, T.; Escudero, J.C. Alelopathic potential of Cistus ladanifer chemicals in response to variations of light and temperature. Chemoecology 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Valares, C.; Sosa, T.; Alías, J.C.; Chaves, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Alías, J.C.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of allelopathy. Allelopath. J. 2016, 38, 113–132. [Google Scholar]
- Jandová, K.; Dostál, P.; Cajthaml, T.; Kameník, Z. Intraspecific variability in allelopathy of Heracleum mantegazzianum is linked to the metabolic profile of root exudates. Ann. Bot. 2014, 115, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Baas, W.J. Secondary plant compounds, their ecological significance and consequences for the carbon budget. Introduction of the carbon/nutrient cycle theory. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; Lambers, H., Cambridge, M.L., Konings, H., Pons, T.L., Eds.; SPB Academic Publishing bv: Hague, The Netherlands, 1989; pp. 313–340. [Google Scholar]
- Chou, C.H.; Kuo, Y.L. Allelopathic research of subtropical vegetation in Taiwan. Allelopathic exclusion of understorey by Leucaena leucophylla (Lam) de Wit. J. Chem. Ecol. 1986, 12, 1431–1448. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Biochemical plant ecology. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1997; pp. 503–516. [Google Scholar]
- Panagopoulos, I.; Bornman, J.F.; Björn, L.O. Response of sugar beet plants to ultraviolet-B (280–320 nm) radiation and Cercospora leaf spot disease. Physiol. Plant. 1992, 84, 140–145. [Google Scholar] [CrossRef]
- Ziska, L.H.; Teramura, A.H.; Sullivan, J.H.; Mccoy, A. Influence of ultraviolet-B (UV-B) radiation on photosynthetic and growth characteristics in field-grown cassava (Manihot esculentum Crantz). Plant. Cell. Environ. 1993, 16, 73–79. [Google Scholar] [CrossRef]
- Croteau, R.; Johnson, M.A. Biosynthesis of terpenoid wood extractives. In Biosyntheis and Biodegradation of Wood Componentes; Higuchi, T., Ed.; Academic Press: Orlando, FL, USA, 1985; pp. 379–439. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Kluwer Academic Publishers: Boston, MA, USA, 1998. [Google Scholar]
- Chitwood, D.J. Nematicidal compounds from plants. In Phytochemical Resources for Medicine and Agriculture; Nigg, H.N., Seigler, D.S., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 185–204. [Google Scholar]
- Chaves, N.; Escudero, J.C. Allelopathic effect of Cistus ladanifer on seed germination. Funct. Ecol. 1997, 11, 432–440. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and effects of the interaction of phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Germination inhibition of herbs in Cistus ladanifer L. soil: Possible involvemente of allelochemicals. Allelopath. J. 2003, 11, 31–42. [Google Scholar]
- Chaves, N.; Escudero, J.C. Variation of flavonoid synthesis induced by ecological factors. In Principles and Practices in Plant Ecology; Dakshini, K.M.N., Chester, F.L., Eds.; Allelochemicals Interactions, CRC Press: Boca Raton, FL, USA, 1999; pp. 267–285. [Google Scholar]
- Vourc’h, G.; Vila, B.; Gillon, D.; Escarré, J.; Guibal, F.; Fritz, H.; Clausen, T.P.; Martin, J.L. Disentangling the causes of damage variation by deer browsing on young Thuja alicata. Oikos 2002, 98, 271–283. [Google Scholar] [CrossRef]
- Karban, R.; Agrawal, A.A.; Mangel, M. The benefits of induced defenses against herbivores. Ecology 1997, 78, 1351–1355. [Google Scholar] [CrossRef]
- Dolch, R.; Tscharntke, T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 2000, 125, 504–511. [Google Scholar] [CrossRef]
- Shelton, A.L. Variable chemical defences in plants and their effects on herbivore behaviour. Evol. Ecol. Res. 2000, 2, 231–249. [Google Scholar]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.L.; Julkunen-Tiitto, R.; Rousi, M. Variation in phenolic compounds within a birch (Betula pendula) population. J. Chem. Ecol. 2000, 26, 1609–1622. [Google Scholar] [CrossRef]
- Laitinen, M.L.; Julkunen-Tiitto, R.; Tahvanainen, J.; Heinonen, J.; Rousi, M. Variation in birch (Betula pendula) shoot secondary chemistry due to genotype, environment and ontogeny. J. Chem. Ecol. 2005, 31, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Morphological and functional types in plants of the Mediterranean scrub of southern Spain. Stvdia Oecol. 1984, 5, 7–34. [Google Scholar]
- Nuñez, E. Jaral Ecology of Cistus ladanifer L. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 1989. [Google Scholar]
- Castroviejo, S.; Aedo, C.; Cirujano, S.; Laínz, M.; Montserrat, P.; Morales, R.; Muñoz Garmendia, F.; Navarro, C.; Paiva, J.; Soriano, C. Flora Iberica 3; Real Jardín Botánico, CSIC: Madrid, Spain, 1993. [Google Scholar]
- Herranz, J.; Ferrandis, P.; Copete, M.A.; Duro, E.M.; Zalacaín, A. Effects of allelopathic compounds produced by Cistus ladanifer on germination of 20 Mediterranean taxa. Plant Ecol. 2006, 184, 259–272. [Google Scholar] [CrossRef]
- Cabezas, J.; Núñez, E.; Escudero, J.C. Spatial and Temporal Distribution of Rainfall in the Province of Badajoz and Quantification of the Volumes of Water Precipitated by Planimetry; Government of Extremadura: Badajoz, Spain, 1989.
- Valares, C. Variación Del Metabolismo Secundario en Plantas Debida al Genotipo Y al Ambiente. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 2011. [Google Scholar]
- Vogth, T.; Gülz, P.G. Isocratic column liquid chromatographic separation of a complex mixture of epicuticular flavonoid aglycones and intracellelar flavonol glycosides from Cistus laurifolius L. J. Chromatogr. 1991, 537, 453–459. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of ecological variables in the seasonal variation of flavonoid content of Cistus ladanifer exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds: apigenin, kaempferol 3-methyl ether, apigenin 4′-methyl ether, apigenin 7-methyl ether and kaempferol 3,7-di-O-methyl ether, 6β-acetoxy-7-oxo-8-labden-15-oic acid, 7-oxo-8-labden-15-oic acid and oxocativic acid are available from the authors.






© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valares Masa, C.; Alías Gallego, J.C.; Chaves Lobón, N.; Sosa Díaz, T. Intra-Population Variation of Secondary Metabolites in Cistus ladanifer L. Molecules 2016, 21, 945. https://doi.org/10.3390/molecules21070945
Valares Masa C, Alías Gallego JC, Chaves Lobón N, Sosa Díaz T. Intra-Population Variation of Secondary Metabolites in Cistus ladanifer L. Molecules. 2016; 21(7):945. https://doi.org/10.3390/molecules21070945
Chicago/Turabian StyleValares Masa, Cristina, Juan Carlos Alías Gallego, Natividad Chaves Lobón, and Teresa Sosa Díaz. 2016. "Intra-Population Variation of Secondary Metabolites in Cistus ladanifer L." Molecules 21, no. 7: 945. https://doi.org/10.3390/molecules21070945