Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors
Abstract
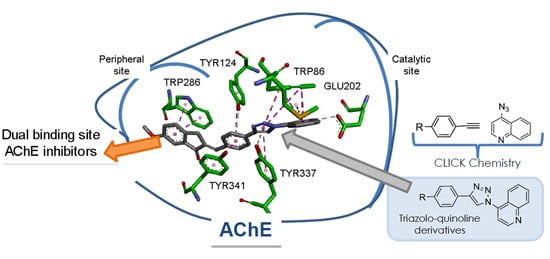
:1. Introduction

2. Results and Discussion
2.1. Chemical Synthesis

2.2. Biological Evaluation
| Compounds | hAChE | hBChE | |||
|---|---|---|---|---|---|
| % Inhibition for Assay hAChE (at 100 μM) | IC50 ± SEM (μM) | Ki ± SEM (μM) | Mechanism Type | % Inhibition for Assay hBChE (at 100 μM) | |
| Tacrine | 92.8 | 1.7 ± 0.1 | 0.07 ± 0.01 | mixed-type | 98.0 |
| Donepezil | 83.9 | 0.12± 0.02 | 2.2 ± 0.05 | mixed-type | 11.1 |
| 3a | 29.0 | 128.0 ± 25 | 185 ± 29 | ND | 0 |
| 3b | 0 | ND | ND | ND | 5.2 |
| 3c | 5.40 | ND | ND | ND | 6.2 |
| 3d | 9.10 | ND | ND | ND | 0 |
| 3e | 37.7 | 146.6 ± 15 | 530 ± 150 | ND | 0 |
| 3g | 48.1 | 114.0 ± 10 | 220 ± 60 | mixed-type | 0.2 |
| 3h | 55.7 | 109.4 ± 14 | 245 ± 70 | mixed-type | 0 |

2.3. Molecular Modeling

3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. 4-Azidoquinoline (5)
3.2.2. 4-(4-(4-Hydroxymethyl-phenyl)-1H-1,2,3-triazol-1-yl)-quinoline (6)
3.2.3. 4-(1-(Quinolin-4-yl)-1H-1,2,3-triazol-1-yl)-benzaldehyde (7)
3.2.4. General Procedure for the Preparation of 3a–h
3.3. Biological Assays
False-Positive Effects on BChE and AChE Inhibition in the Thin Layer Chromatography (TLC) Assay Based on Ellman’s Method
3.4. Docking Procedures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, D.; Dorronsoro, I.; Rubio, L.; Munõz, P.; García-Palomero, E.; del Monte, M.; Bidon-Chanal, A.; Orozco, M.; Luque, F.J.; Castro, A.; et al. Donepezil-tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg. Med. Chem. 2005, 13, 6588–6597. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can. J. Psychiatry 2014, 59, 618–623. [Google Scholar] [PubMed]
- Inestrosa, N.C.; Dinamarca, M.C.; Alvarez, A. Amyloid-cholinesterase interactions. Implications for Alzheimer’s disease. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Martinez, A. Peripheral and dual binding site acetylcholinesterase inhibitors: Implications in treatment of Alzheimer’s disease. Mini Rev. Med. Chem. 2001, 1, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Martinez, A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Camps, G.P.; Formosa, M.X.; Muñoz-Torrero, L.I.D.; Scarpellini, M. Acetylcholinesterase-Inhibiting Compounds for Treating Alzheimer Desease. WO 2007/122274 A1, 1 November 2007. [Google Scholar]
- Camps, P.; Formosa, X.; Galdeano, C.; Gómez, T.; Muñoz-Torrero, D.; Scarpellini, M.; Viayna, E.; Badia, A.; Clos, M.V.; Camins, A.; et al. Novel Donepezil-Based Inhibitors of Acetyl- and Butyrylcholinesterase and Acetylcholinesterase-Induced -Amyloid Aggregation. J. Med. Chem. 2008, 51, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; Formosa, X.; Galdeano, C.; Gómez, T.; Muñoz-Torrero, D.; Ramírez, L.; Viayna, E.; Gómez, E.; Isambert, N.; Lavilla, R.; et al. Tacrine-based dual binding site acetylcholinesterase inhibitors as potential disease-modifying anti-Alzheimer drug candidates. Chem. Biol. Interact. 2010, 187, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Ruiz, P.; Rubio, L.; García-Palomero, E.; Dorronsoro, I.; del Monte-Mil, M.; Valenzuela, R.; Usán, P.; de Austria, C.; Bartolini, M.; Andrisano, V.; et al. Design, Synthesis, and Biological Evaluation of Dual Binding Site Acetylcholinesterase Inhibitors: New Disease-Modifying Agents for Alzheimer’s Disease. J. Med. Chem. 2005, 48, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Luo, W.; Li, Y.; He, Y.; Huang, S.; Tan, J.; Ou, T.; Li, D.; Gu, L.; Huang, Z. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as dual inhibitors for cholinesterases and amyloid beta aggregation. Bioorg. Med. Chem. 2011, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zou, C.; Luo, C.; Tang, X.; Li, Y. Synthesis and evaluation of tacrine-E2020 hybrids as acetylcholinesterase inhibitors for the treatment of Alzheimer´s disease. Bioorg. Med. Chem. Lett. 2004, 14, 4639–4642. [Google Scholar] [CrossRef] [PubMed]
- Korabecny, J.; Dolezal, R.; Cabelova, P.; Horova, A.; Hruba, E.; Ricny, J.; Sedlacek, L.; Nepovimova, E.; Spilovska, K.; Andrs, M.; et al. 7-MEOTAedonepezil like compounds as cholinesterase inhibitors: Synthesis, pharmacological evaluation, molecular modeling and QSAR Studies. Eur. J. Med. Chem. 2014, 82, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.R.; Deschênes, S.M.; Manyin, T.; Fuchs, R.P.P. Mutagenicity of acridines in a reversion assay based on tetracycline resistance in plasmid pBR322 in Escherichia coli. Mutat. Res. 1996, 351, 33–43. [Google Scholar] [CrossRef]
- Marella, A.; Tanwar, O.P.; Saha, R.; Ali, M.R.; Srivastava, S.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Krasiński, A.; Radić, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K.B.; Kolb, H.C. In situ selection of lead compounds by click chemistry: Target-guided optimization of acetylcholinesterase inhibitors. J. Am. Chem. Soc. 2005, 127, 6686–6692. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Huang, L.; Lu, C.; He, F.; Li, X. Synthesis, biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and β-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011, 19, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Khanaposhtani, M.; Saeedi, M.; Zafarghandi, N.S.; Mahdavi, M.; Sabourian, R.; Razkenari, E.K.; Alinezhad, H.; Khanavi, M.; Foroumadi, A.; Shafiee, A.; et al. Potent acetylcholinesterase inhibitors: Design, synthesis, biological evaluation, and docking study of acridone linked to 1,2,3-triazole derivatives. Eur. J. Med. Chem. 2015, 92, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Chioua, M.; Samadi, A.; Agostinho, P.; Garção, P.; Lajarín-Cuesta, R.; de los Ríos, C.; Iriepa, I.; Moraleda, I.; Gonzalez-Lafuente, L.; et al. Synthesis, Pharmacological Assessment, and Molecular Modeling of Acetylcholinesterase/Butyrylcholinesterase Inhibitors: Effect against Amyloid-β-Induced Neurotoxicity. ACS Chem. Neurosci. 2013, 4, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.T.; Marquez, B.L.; Gerwick, W.H.; Kövér, K. One- and two-dimensional gradient-selected HSQMBC NMR experiments for the efficient analysis of long-range heteronuclear coupling constants. Magn. Reson. Chem. 2000, 38, 265–273. [Google Scholar] [CrossRef]
- Lacerda, V., Jr.; da Silva, G.V.; Constantino, M.G.; Tormena, C.F.; Williamson, R.T.; Marquez, B.L. Long-range JCH heteronuclear coupling constants in cyclopentane derivatives. Magn. Reson. Chem. 2006, 44, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, V., Jr.; da Silva, G.V.; Constantino, M.G.; Tormena, C.F.; Williamson, R.T.; Marquez, B.L. Long-range JCH heteronuclear coupling constants in cyclopentane derivatives. Part II. Magn. Reson. Chem. 2007, 45, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, V., Jr.; da Silva, G.V.; Constantino, M.G.; dos Santos, R.B.; de Castro, E.V.; Silva, R.C. Stereochemistry of cyclopentane derivatives from 2,3JCH dependence on dihedral angle. Magn. Reson. Chem. 2008, 46, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Mohrig, J.R.; Alberg, D.G.; Cartwright, C.H.; Pflum, M.K.; Aldrich, J.S.; Anderson, J.K.; Anderson, S.R.; Fimmen, R.L.; Snover, A.K. Stereochemistry of 1,2-elimination reactions at the E2-E1cB interface-tert-butyl 3-tosyloxybutanoate and its thioester. Org. Biomol. Chem. 2008, 6, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Khorana, N.; Markmee, S.; Ingkaninan, K.; Ruchirawat, S.; Kitbunnadaj, R.; Pullagurla, M.R. Evaluation of a new lead for acetylcholinesterase inhibition. Med. Chem. Res. 2009, 18, 231–241. [Google Scholar] [CrossRef]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wu, P.; Yang, R.; Gao, L.; Li, C.; Wang, D.; Wu, S.; Liu, A.L.; Du, G.H. Inhibition of acetylcholinesterase by two genistein derivatives: Kinetic analysis, molecular docking and molecular dynamics simulation. Acta Pharm. Sin. B 2014, 4, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Alhomida, A.S.; Al-Rajhi, A.A.; Kamal, M.A.; Al Jafari, A.A. Kinetic analysis of the toxicological effect of tacrine (Cognex®) on human retinal acetylcholinesterase activity. Toxicology 2000, 147, 33–39. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Liebeschuetz, J.W.; Cole, J.C.; Korb, O. Pose prediction and virtual screening performance of GOLD scoring functions in a standardized test. J. Comput. Aided Mol. Des. 2012, 26, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2003. [Google Scholar]
- García-Palomero, E.; Muñoz, P.; Usan, P.; Garcia, P.; Delgado, E.; de Austria, C.; Valenzuela, R.; Rubio, L.; Medina, M.; Martínez, A. Potent beta-amyloid modulators. Neurodegener. Dis. 2008, 5, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantoani, S.P.; Chierrito, T.P.C.; Vilela, A.F.L.; Cardoso, C.L.; Martínez, A.; Carvalho, I. Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors. Molecules 2016, 21, 193. https://doi.org/10.3390/molecules21020193
Mantoani SP, Chierrito TPC, Vilela AFL, Cardoso CL, Martínez A, Carvalho I. Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors. Molecules. 2016; 21(2):193. https://doi.org/10.3390/molecules21020193
Chicago/Turabian StyleMantoani, Susimaire P., Talita P. C. Chierrito, Adriana F. L. Vilela, Carmen L. Cardoso, Ana Martínez, and Ivone Carvalho. 2016. "Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors" Molecules 21, no. 2: 193. https://doi.org/10.3390/molecules21020193