Tetrabutylammonium Bromide Media Aza-Michael Addition of 1,2,3,6-Tetrahydrophthalimide to Symmetrical Fumaric Esters and Acrylic Esters under Solvent-Free Conditions
Abstract
:1. Introduction

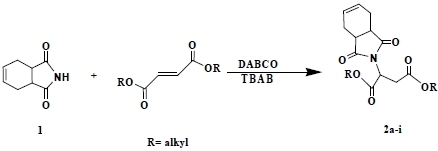
2. Results and Discussion
| Entry | Solventa | Base | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | DMSO | Na2CO3 | 24 | 10 |
| 2 | DMSO | K2CO3 | 24 | 15 |
| 3 | DMSO | Triethylamine | 24 | 10 |
| 4 | DMSO | Pyridine | 24 | 12 |
| 5 | DMSO | DABCO | 24 | 23 |
| 6 | Acetone | Na2CO3 | 24 | - |
| 7 | Acetone | K2CO3 | 24 | - |
| 8 | Acetone | Triethylamine | 24 | - |
| 9 | Acetone | Pyridine | 24 | - |
| 10 | Acetone | DABCO | 24 | 17 |
| 11 | DMF | Na2CO3 | 24 | 10 |
| 12 | DMF | K2CO3 | 24 | 10 |
| 13 | DMF | Triethylamine | 24 | 16 |
| 14 | DMF | Pyridine | 24 | 10 |
| 15 | DMF | DABCO | 24 | 20 |
| 16 | TBABb | Na2CO3 | 24 | 30 |
| 17 | TBABb | K2CO3 | 24 | 40 |
| 18 | TBABb | Triethylamine | 24 | 25 |
| 19 | TBABb | Pyridine | 24 | 18 |
| 20 | TBABb | DABCO | 2:5 | 85 |
| 21 | Nonec | Na2CO3 | 24 | - |
| 22 | Nonec | K2CO3 | 24 | - |
| 23 | Nonec | Triethylamine | 24 | - |
| 24 | Nonec | Pyridine | 24 | - |
| 25 | Nonec | DABCO | 24 | - |
| Entry | Ester | Product | Time(h) | Yield(%)a,b |
|---|---|---|---|---|
| 1 |  |  | 2.0 | — |
| 2 |  |  | 2.5 | 85 |
| 3 |  |  | 3.0 | — |
| 4 |  |  | 3.5 | 76 |
| 5 |  |  | 3.5 | 72 |
| 6 |  |  | 4.0 | 70 |
| 7 |  |  | 4.5 | 68 |
| 8 |  |  | 5.0 | 63 |
| 9 |  |  | 5.5 | 60 |
| 10 |  |  | 6.0 | 57 |
| 11 |  |  | 6.5 | 55 |
| 12 |  |  | 7.0 | — |
| 13 |  |  | 1.5 | 90 |
| 14 |  |  | 24.0 | — |
| 15 |  |  | 24.0 | — |
3. Conclusions
4. Experimental
4.1. General
4.2. General procedure for Michael addition of 1,2,3,6-tetrahydrophthalimide to symmetrical fumaric esters
- Sample Availability: Contact the authors.
References
- Anastas, P.T.; Warner, J.C. Green Chemistry, Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Polshettiwar, V.; Varma, R. S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41, 629. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R. S. Aqueous microwave chemistry: A clean and green synthetic tool for rapid drug discovery. Chem. Soc. Rev. 2008, 37, 1546. [Google Scholar]
- Toda, F.; Tanaka, K. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025. [Google Scholar]
- Li, C.J.; Chen, L. Organic chemistry in water. Chem. Soc. Rev. 2006, 35, 68. [Google Scholar]
- Ranu, B.C.; Banerjee, S. Significant rate acceleration of the aza-Michael reaction in water. Tetrahedron Lett. 2007, 48, 141. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, R.; Chakraborti, A.K. On water’ synthesis of 2,4-diaryl-2,3-dihydro-1,5- benzothiazepines catalysed by sodium dodecyl sulfate (SDS). Tetrahedron Lett. 2008, 49, 4269. [Google Scholar] [CrossRef]
- Khatik, G.L.; Kumar, R.; Chakraborti, A.K. Catalyst-free conjugated addition of thiols to α,β-unsaturated carbonyl compounds in water. Org. Lett. 2006, 8, 2433. [Google Scholar]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2399. [Google Scholar] [CrossRef]
- Chankeshwara, S.V.; Chakraborti, A.K. Catalyst-free chemoselective N-tert-butyloxycarbonylation of amines in water. Org. Lett. 2006, 8, 3259. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Zang, Q. S.; Oderaotoshi, Y.; Curran, D.P. Fluorous mixture synthesis: A fluorous-tagging strategy for the synthesis and separation of mixtures of organic compounds. Science 2001, 291, 1766. [Google Scholar]
- Harvath, I.T. Fluorous biphase chemistry. Acc. Chem. Res. 1998, 31, 641. [Google Scholar] [CrossRef]
- Oakes, R.S.; Califforrd, A.A.; Rayner, C.M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc., Perkin Trans. I 2001, 917. [Google Scholar]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Aqueous polyethylene glycol solutions as green reaction media. Green Chem. 2005, 7, 64. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yin, L.; Wang, Y.M.; Liu, J.Y.; Li, Y. Indium tribromide in poly (ethylene glycol)(PEG): a novel and efficient recycle system for chemoselective deprotection of 1, 1-diacetates. Green Chem. 2004, 6, 563. [Google Scholar] [CrossRef]
- Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon Press: Oxford, UK, 1992. [Google Scholar]
- Hayashi, Y.; Rohde, J.J.; Corey, E.J. A novel super-Lewis acidic catalyst for enantioselective synthesis. J. Am. Chem. Soc. 1996, 118, 5502. [Google Scholar] [CrossRef]
- Kummaraja, M.; Pitchumani, K. Hetero-Michael addition of benzenethiol to cycloalkenones using cation-exchanged faujasites: simultaneous acid–base bifunctional catalysis. J. Mol. Catal. A: Chem. 2006, 256, 138. [Google Scholar] [CrossRef]
- Vicario, J.L.; Badia, D.; Carrillo, L. Asymmetric synthesis of β-substituted α-methyl-β-amino esters by mannich reaction of (S,S)-(+)-pseudoephedrine acetamide derived enolate with imine. Org. Lett. 2001, 3, 773. [Google Scholar] [CrossRef]
- Gellman, S. Foldmers: A manifesto. Acc. Chem. Res. 1998, 31, 173. [Google Scholar] [CrossRef]
- Basu, B.; Das, P.; Hossain, I. Synthesis of β-amino esters via aza-Michael addition of amines to alkenes promoted on silica: A useful and recyclable surface. Synlett 2004, 2630. [Google Scholar]
- Yang, L.; Xu, L.W.; Zhou, W.; Li, L.; Xia, C.G. Highly efficient aza-Michael reactions of aromatic amines and N-heterocycles catalyzed by a basic ionic liquid under solvent-free conditions. Tetrahedron Lett. 2006, 47, 7723. [Google Scholar] [CrossRef]
- Kawatsura, M.; Hartwig, J. F. Transition metal-catalyzed addition of amines to acrylic acid derivatives. A high-throughput method for evaluating hydroamination of primary and secondary alkylamines. Organometallics 2001, 20, 1960. [Google Scholar]
- Srivastava, N.; Banik, B. K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109. [Google Scholar] [CrossRef]
- Varala, R.; Alam, M.M.; Adapa, S.R. Michael type addition of aliphatic amines to α,β-ethylenic compounds using bismuth triflate catalyst. Synlett 2003, 720. [Google Scholar]
- Cao, Y.J.; Lai, Y.Y.; Wang, X.; Li, Y.J.; Xiao, W.J. Michael additions in water of ketones to nitroolefins catalyzed by readily tunable and bifunctional pyrrolidine–thiourea organocatalysts. Tetrahedron Lett. 2007, 48, 21. [Google Scholar]
- Steves, A.P.; Silva, M.E.; Rodrigues, L.M.; Oliveria-Campos, A.M.F.; Hrdina, R. Aza-Michael reactions with vinyl sulfones and Amberlyst-15 as catalyst. Tetrahedron Lett. 2007, 48, 9040. [Google Scholar]
- Verma, A.K.; Kumar, R.; Chaudhary, P.; Saxena, A.; Shankar, R.; Mozumdar, S.; Chandra, R. Cu-nanoparticles: A chemoselective catalyst for the aza-Michael reactions of N-alkyl- and N-arylpiperazines with acrylonitrile. Tetrahedron Lett. 2005, 46, 5229. [Google Scholar] [CrossRef]
- Mariella, R.; Jonauskas, R. Cyanoethylation of aromatic amides. J. Org. Chem. 1958, 23, 923. [Google Scholar] [CrossRef]
- Maggini, M.; Prato, M.; Ranelli, M.; Scorrano, G. Synthesis of (−)-8-deoxy-7-hydroxy-swainsonine and (±)-6,8-dideoxy-castanospermine. Tetrahedron Lett. 1992, 33, 6537. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Oerz, R. 1,4-Addition reactions to methacrylamide : A one pot synthesis of 3,4-dihydro 2(1H)-pyridinones and 3,5-disubstituted glutarimides. Tetrahedron Lett. 1985, 26, 1311. [Google Scholar] [CrossRef]
- Bredereck, H.; Gompper, R.; Herlinger, H.; Wotiun, E. Säureamid-Reaktionen, XXIV. umsetzungen von formamid mit Mannich-basen. Chem. Ber. 1960, 93, 2423. [Google Scholar] [CrossRef]
- Reitz, A.; Verlander, M.; Goodman, M. Alumina catalyzed transformations of O-(3-oxobutyl) urethanes. Tetrahedron Lett. 1982, 23, 751. [Google Scholar] [CrossRef]
- Imanzadeh, G.H.; Khalafinezhad, A.; Zare, A.; Hasaninejad, A.; Mosavi Zare, A.; Parhami, A. Michael addition of phthalimid and saccharin to α,β-unsaturated esters under solvent-free conditions. J. Iran. Chem. Soc. 2007, 4, 229. [Google Scholar] [CrossRef]
- Imanzadeh, I.G.; Tavana, M.M.; Zamanloo, M.R.; Mansoori, Y. Aza-Michael addition of isatin and phthalimide to symmetrical fumaric esters in ionic liquid media. Chin. J. Chem. 2009, 27, 389. [Google Scholar]
- Vogel, A. Vogel,s Practical Organic Chemistry, 4th ed.; Longman,Press: London, UK, 1978. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imanzadeh, G.; Ahmadi, F.; Zamanloo, M.; Mansoori, Y. Tetrabutylammonium Bromide Media Aza-Michael Addition of 1,2,3,6-Tetrahydrophthalimide to Symmetrical Fumaric Esters and Acrylic Esters under Solvent-Free Conditions. Molecules 2010, 15, 7353-7362. https://doi.org/10.3390/molecules15107353
Imanzadeh G, Ahmadi F, Zamanloo M, Mansoori Y. Tetrabutylammonium Bromide Media Aza-Michael Addition of 1,2,3,6-Tetrahydrophthalimide to Symmetrical Fumaric Esters and Acrylic Esters under Solvent-Free Conditions. Molecules. 2010; 15(10):7353-7362. https://doi.org/10.3390/molecules15107353
Chicago/Turabian StyleImanzadeh, Gholamhassan, Farzaneh Ahmadi, Mohammadreza Zamanloo, and Yagoub Mansoori. 2010. "Tetrabutylammonium Bromide Media Aza-Michael Addition of 1,2,3,6-Tetrahydrophthalimide to Symmetrical Fumaric Esters and Acrylic Esters under Solvent-Free Conditions" Molecules 15, no. 10: 7353-7362. https://doi.org/10.3390/molecules15107353