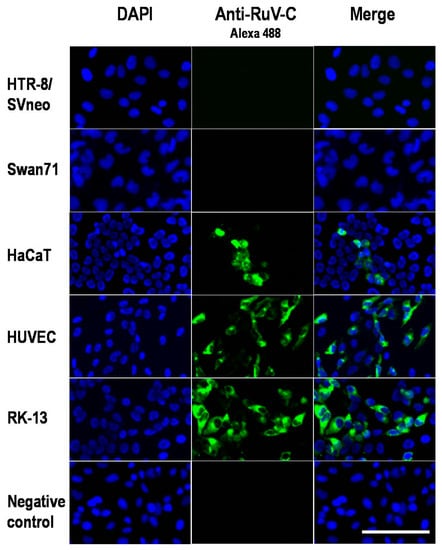
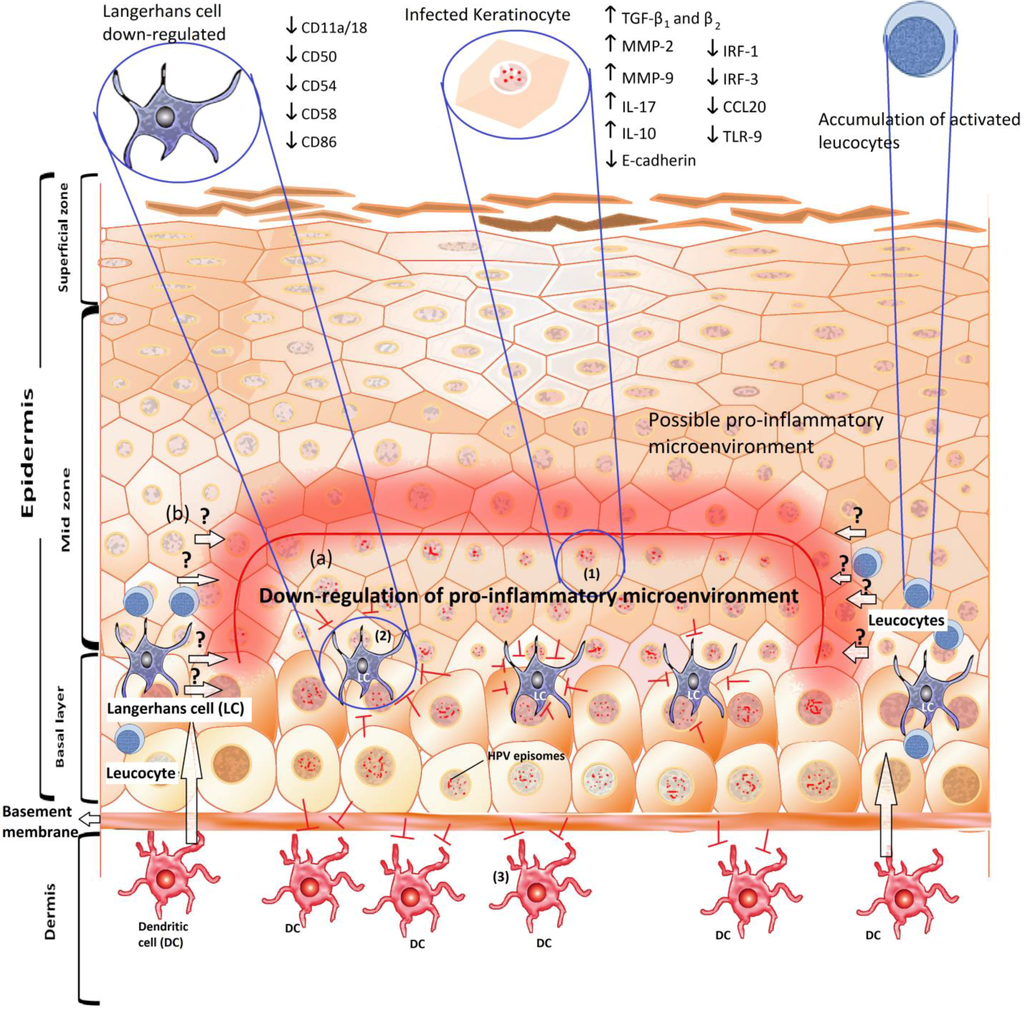
Global Viral Threats
A topical collection in Viruses (ISSN 1999-4915).
Editor
Interests: HIV dynamics and replication
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
In this Topical Collection of Viruses, we offer a platform to submit original research articles on the very latest themes in virology. In particular, we encourage submissions concerning recently identified strains with the potential to pose a widespread threat to animals, crops, or public health. With a rapid publication time of 40 days, Viruses is uniquely poised for the rapid dissemination of such knowledge.
Eric O. Freed
Editor-in-Chief
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).