Journal Description
Transplantology
Transplantology
is an international, peer-reviewed, open access journal on all areas of experimental and clinical transplantation, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 40.2 days after submission; acceptance to publication is undertaken in 5.7 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Transplantology is a companion journal of JCM.
Latest Articles
Robotic Surgical Techniques in Transplantation: A Comprehensive Review
Transplantology 2024, 5(2), 72-84; https://doi.org/10.3390/transplantology5020008 (registering DOI) - 25 Apr 2024
Abstract
In the field of surgery, the idea of performing organ transplants in a minimally invasive fashion has always been a significant technical challenge. The advent of the robotic approach facilitated the overcoming of difficulties in highly complex surgical procedures that demand high technical
[...] Read more.
In the field of surgery, the idea of performing organ transplants in a minimally invasive fashion has always been a significant technical challenge. The advent of the robotic approach facilitated the overcoming of difficulties in highly complex surgical procedures that demand high technical skill. Furthermore, robotic transplants are showing significant benefits in patient outcomes, particularly in the obese population. The purpose of this review is to provide an overview of the current state of robotics applications for transplant surgery. Kidney transplants were the first to be performed using a fully robotic approach. Since then, robotic surgery has gradually been applied to other organ transplants, with very recent reports of fully robotic lung and liver transplants. Further experiences and studies will be needed to verify their effectiveness and to satisfy some concerns regarding the longer warm ischemia time related to the robotic approach in comparison with open surgery.
Full article
Open AccessCase Report
From Normal Renal Function to Renal Replacement Therapy after Liver Transplantation: A Case Report
by
Samuel Mangold, Gergely Albu, Julien Maillard, Florence Aldenkortt and Eduardo Schiffer
Transplantology 2024, 5(2), 65-71; https://doi.org/10.3390/transplantology5020007 - 11 Apr 2024
Abstract
Postoperative renal failure significantly impacts long-term renal function and the overall survival of patients receiving liver transplantation (LT), being a crucial factor in their morbidity and mortality. It is difficult to define whether the causes of renal failure are solely related to surgery
[...] Read more.
Postoperative renal failure significantly impacts long-term renal function and the overall survival of patients receiving liver transplantation (LT), being a crucial factor in their morbidity and mortality. It is difficult to define whether the causes of renal failure are solely related to surgery or anaesthesia during liver transplantation (LT). Indeed, liver disease requiring liver transplantation is often the cause of preoperative renal failure. We report a case of a 62-year-old patient undergoing LT for cholangiocarcinoma that led to acute kidney injury postoperatively while his preoperative renal function was normal. This report highlights the major influence that the surgical and anaesthetic procedure can have on renal function and identifies the factors that may have led to renal replacement therapy being required for this patient.
Full article
(This article belongs to the Collection Progress and Recent Advances in Solid Organ Transplantation)
Open AccessArticle
Wasted Potential: Decoding the Trifecta of Donor Kidney Shortage, Underutilization, and Rising Discard Rates
by
Ceilidh McKenney, Julia Torabi, Rachel Todd, M. Zeeshan Akhtar, Fasika M. Tedla, Ron Shapiro, Sander S. Florman, Matthew L. Holzner and L. Leonie van Leeuwen
Transplantology 2024, 5(2), 51-64; https://doi.org/10.3390/transplantology5020006 - 28 Mar 2024
Abstract
►▼
Show Figures
Kidney transplantation is a life-saving intervention for end-stage renal disease; yet, the persistent gap between organ demand and supply remains a significant challenge. This paper explores the escalating discard rates of deceased donor kidneys in the United States to assess trends, discard reasons,
[...] Read more.
Kidney transplantation is a life-saving intervention for end-stage renal disease; yet, the persistent gap between organ demand and supply remains a significant challenge. This paper explores the escalating discard rates of deceased donor kidneys in the United States to assess trends, discard reasons, demographical differences, and preservation techniques. Data from the Scientific Registry of Transplant Recipients from 2010 to 2021 was analyzed using chi-squared tests for trend significance and logistic regression to estimate odds ratios for kidney discard. Over the last decade, discard rates have risen to 25% in 2021. Most discarded kidneys came from extended criteria donor (ECD) donors and elevated kidney donor profile index (KDPI) scores. Kidney biopsy status was a significant factor and predictor of discard. Discard rates varied greatly between Organ Procurement and Transplantation Network regions. Of reasons for discard, “no recipient located” reached a high of 60%. Additionally, there has been a twofold increase in hypothermic machine perfusion (HMP) since 2010, with transportation difficulties being the main reason for the discard of perfused kidneys. Our findings suggest a need to recalibrate organ utilization strategies, optimize the use of lower-quality kidneys through advanced preservation methods, and address the evolving landscape of organ allocation policies to reduce kidney discard rates.
Full article
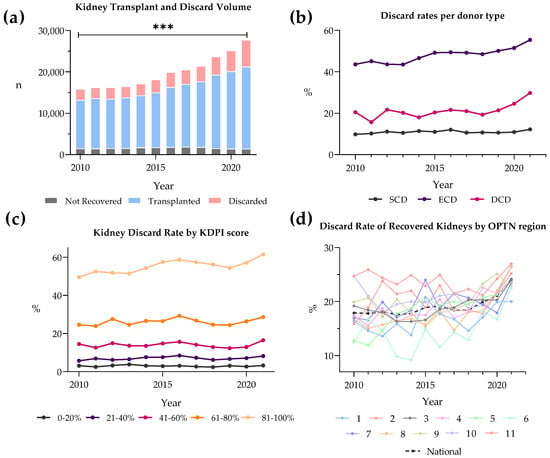
Figure 1
Open AccessBrief Report
Novel Study of SARS-CoV-2 RNA in Post-Reperfusion Liver Biopsies after Transplantation Using COVID-19-Positive Donor Allografts
by
Jenna N. Whitrock, Michela M. Carter, Adam D. Price, Aaron M. Delman, Catherine G. Pratt, Jiang Wang, Divya Sharma, Ralph C. Quillin III and Shimul A. Shah
Transplantology 2024, 5(1), 46-50; https://doi.org/10.3390/transplantology5010005 - 16 Mar 2024
Abstract
The utilization of COVID-19-positive donors has expanded the donor pool for transplantation since the initiation of COVID allograft utilization protocols. However, the biopsy-proven PCR transmission rate of COVID-19 from these allografts has not been well documented. In August 2021, an institutional COVID-19-positive allograft
[...] Read more.
The utilization of COVID-19-positive donors has expanded the donor pool for transplantation since the initiation of COVID allograft utilization protocols. However, the biopsy-proven PCR transmission rate of COVID-19 from these allografts has not been well documented. In August 2021, an institutional COVID-19-positive allograft protocol was implemented for liver and kidney transplants. Post-reperfusion liver biopsies were obtained intra-operatively to evaluate for COVID-19 RNA, and post-operative day 7 nasopharyngeal reverse transcriptase polymerase chain reaction (RT-PCR) swabs were collected. The primary endpoints evaluated included COVID-19 RNA on biopsy and COVID-19 detected via nasopharyngeal RT-PCR swab on post-operative day 7. A total of 20 vaccinated recipients underwent transplantation (17 liver only, 3 simultaneous liver and kidney) with whole liver allografts from 20 COVID-19-positive deceased donors between August 2021 and April 2022. 95% (19/20) of donors were asymptomatic at the time of donation. On post-reperfusion liver allograft biopsies, COVID-19 RNA was found in 10% (2/20) of the samples. All the recipients were COVID-19-negative on post-operative day 7 nasopharyngeal RT-PCR, showing a 0% transmission rate of COVID-19 from the positive allografts. The use of COVID-19 allografts appears to be a safe practice, with no PCR-detectable transmission of COVID-19 despite 10% of the liver allografts having COVID-19 RNA present on post-reperfusion biopsy.
Full article
Open AccessArticle
Pre-Emptive Kidney Retransplantation from Deceased Donors
by
Antonio Franco Esteve, Patricio Mas-Serrano, Fransico Manuel Marco, Eduardo Garin Cascales and Francisco Javier Perez Contreras
Transplantology 2024, 5(1), 37-45; https://doi.org/10.3390/transplantology5010004 - 28 Feb 2024
Abstract
There is uncertainty about the best approach to replacement treatment for kidney transplant recipients with chronic terminal graft dysfunction, since a retransplant could be performed before the resumption of dialysis, thus avoiding this treatment and the dilemma of whether or not to suspend
[...] Read more.
There is uncertainty about the best approach to replacement treatment for kidney transplant recipients with chronic terminal graft dysfunction, since a retransplant could be performed before the resumption of dialysis, thus avoiding this treatment and the dilemma of whether or not to suspend immunosuppressive therapy. However, there is limited experience in pre-emptive repeat transplantations, and none from deceased donors. This study aims to assess the results of a pre-emptive retransplantation program with brain-dead deceased donors. We designed a retrospective matched cohort study, including 36 recipients in the pre-dialysis group and 36 controls who were already on dialysis, matched for donor age and transplant date, which could not differ by more than 7 days between pairs. The variables used to standardize the cohorts were donor and recipient age and sex, blood group, duration of the first graft, time on the waitlist to receive the second graft, cold ischemia time, induction and maintenance of immunosuppression, and HLA antibodies (-) prior to retransplantation. The efficacy variables were early graft loss, acute rejection, delay in graft function, renal function at the end of follow-up, survival time, and recipient and graft survival at 24 and 48 months’ follow-up. The pre-dialysis group presented a significantly shorter waitlist time, lower immunization status, and a significantly longer duration of the first graft than the control group. The percentage of recipients who presented early graft loss, delayed renal function, or acute rejection was similar between groups. No significant differences were observed in kidney function or in the survival of the recipient or graft. Retransplantation yields good outcomes in patients with terminal chronic dysfunction, helping to avoid recurrence to dialysis, shortening the time spent on the waitlist, reducing the risk of producing antibodies, and resolving the dilemma of whether or not to stop immunosuppression.
Full article
(This article belongs to the Section Solid Organ Transplantation)
►▼
Show Figures
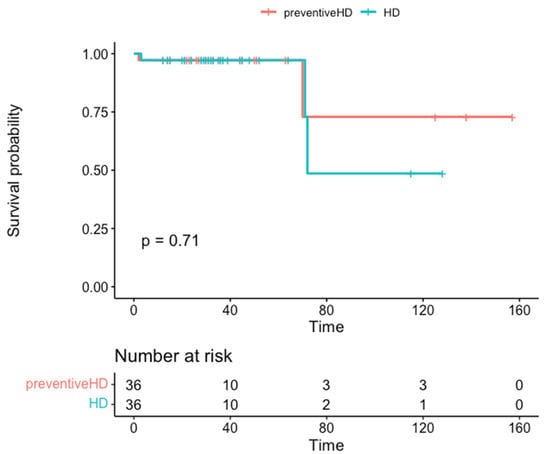
Figure 1
Open AccessArticle
Availability of Deceased Donors for Uterus Transplantation in the United States: Perception vs. Reality
by
Kathleen O’Neill, Elliott G. Richards, Jessica Walter, Sharon West, Richard Hasz, Giuliano Testa, Shreya Kalra, Tommaso Falcone, Rebecca Flyckt, Nawar Latif, Andreas Tzakis and Liza Johannesson
Transplantology 2024, 5(1), 27-36; https://doi.org/10.3390/transplantology5010003 - 04 Feb 2024
Abstract
►▼
Show Figures
Uterus transplantation (UTx) is a rapidly evolving treatment for uterine factor infertility. New centers offering this treatment must decide whether to utilize living donors, deceased donors, or both. Although limiting UTx to deceased donors eliminates the surgical risks for living donors, an adequate
[...] Read more.
Uterus transplantation (UTx) is a rapidly evolving treatment for uterine factor infertility. New centers offering this treatment must decide whether to utilize living donors, deceased donors, or both. Although limiting UTx to deceased donors eliminates the surgical risks for living donors, an adequate supply of suitable deceased uterus donors in the United States is an emerging concern. Previous studies describing the paucity of deceased uterus donors failed to consider key donor characteristics, potentially overestimating the available organ pool. To estimate the United States’ supply of deceased donor uteri; we extrapolated detailed clinical and demographic information from the regional donor datasets available from three organ procurement organizations to the national Organ Procurement and Transplantation Network donor pool. We estimate there are approximately 3700 possible and 400 optimal uterus donors annually in the United States. Given these projections and the number of women with uterine factor infertility in the U.S. who pursue parenthood through alternative strategies, we conclude that, as uterus transplant transitions from research to established clinical care, demand could quickly exceed the deceased donor supply. The liberalization of deceased donor selection criteria may be insufficient to address this imbalance; therefore, fulfilling the anticipated increased demand for uterus transplantation may require and justify greater use of living donors.
Full article
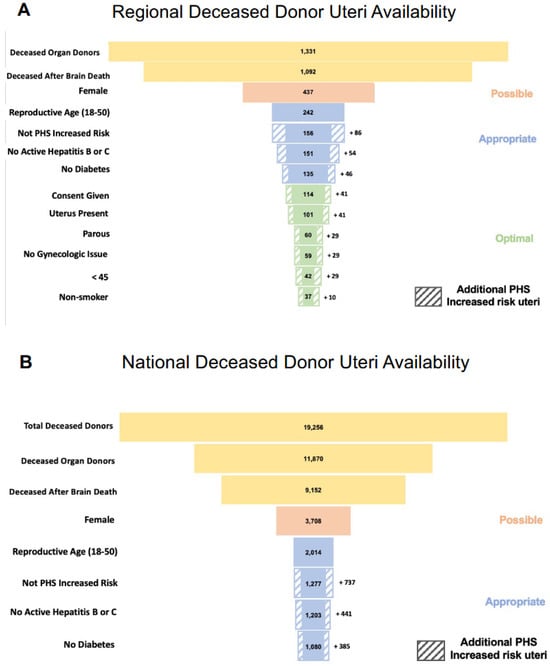
Figure 1
Open AccessArticle
Personality Changes Associated with Organ Transplants
by
Brian Carter, Laveen Khoshnaw, Megan Simmons, Lisa Hines, Brandon Wolfe and Mitchell Liester
Transplantology 2024, 5(1), 12-26; https://doi.org/10.3390/transplantology5010002 - 17 Jan 2024
Abstract
Personality changes have been reported following organ transplantation. Most commonly, such changes have been described among heart transplant recipients. We set out to examine whether personality changes occur following organ transplantation, and specifically, what types of changes occur among heart transplant recipients compared
[...] Read more.
Personality changes have been reported following organ transplantation. Most commonly, such changes have been described among heart transplant recipients. We set out to examine whether personality changes occur following organ transplantation, and specifically, what types of changes occur among heart transplant recipients compared to other organ recipients. A cross-sectional study was conducted in which 47 participants (23 heart recipients and 24 other organ recipients) completed an online survey. In this study, 89% of all transplant recipients reported personality changes after undergoing transplant surgery, which was similar for heart and other organ recipients. The only personality change that differed between heart and other organ recipients and that achieved statistical significance was a change in physical attributes. Differences in other types of personality changes were observed between these groups but the number of participants in each group was too small to achieve statistical significance. Overall, the similarities between the two groups suggest heart transplant recipients may not be unique in their experience of personality changes following transplantation, but instead such changes may occur following the transplantation of any organ. With the exception of physical attributes, the types of personality changes reported were similar between the two groups. These finding indicate that heart transplant recipients are not unique in their reported experience of personality changes following organ transplantation. Further studies are needed to deepen our understanding of what causes these personality changes.
Full article
(This article belongs to the Collection Progress and Recent Advances in Solid Organ Transplantation)
►▼
Show Figures
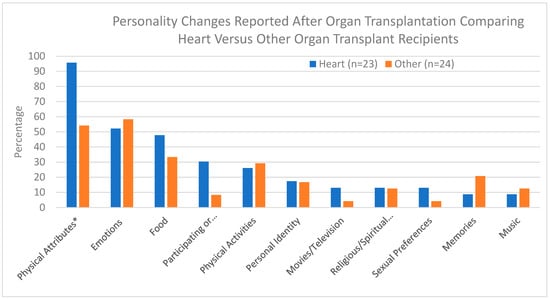
Figure 1
Open AccessReview
Frailty as a Prognostic Indicator in Lung Transplantation: A Comprehensive Analysis
by
René Hage and Macé Matthew Schuurmans
Transplantology 2024, 5(1), 1-11; https://doi.org/10.3390/transplantology5010001 - 19 Dec 2023
Abstract
Introduction: Frailty is a complex pathobiological process characterized by diminished physiological reserve and increased vulnerability to stressors, which has been associated with unfavorable outcomes before and after lung transplantation. Methods: We undertook an extensive narrative review, encompassing a thorough exploration of original papers,
[...] Read more.
Introduction: Frailty is a complex pathobiological process characterized by diminished physiological reserve and increased vulnerability to stressors, which has been associated with unfavorable outcomes before and after lung transplantation. Methods: We undertook an extensive narrative review, encompassing a thorough exploration of original papers, observational studies, case reports, and meta-analyses published between 1990 and July 2023, in various databases, including PubMed, Embase, Cochrane Library, Wiley Online Library databases, and Google Scholar. The search terms [frailty] AND [lung transplant] were utilized. Additionally, the reference lists of retrieved articles were examined. Inclusion criteria comprised studies written in English and involving human subjects. The identified studies were categorized into pre-transplant and post-transplant populations, and the measurement tools used to assess frailty were analyzed, along with the clinical implications reported in the studies. Results: From 1 January 1990 to 1 July 2023, a total of 10 studies on frailty and lung transplantation were identified through online sources and bibliographic searches, involving a total of 2759 patients. Among these studies, six focused on the pre-transplant population, while four examined the post-transplant population. The Fried Frailty Phenotype (FFP) and the Short Physical Performance Battery (SPPB) were the most employed tools for measuring frailty. A table presents additional frailty assessment instruments and the clinical implications described in the studies. Conclusions: Frailty is prevalent both in patients with end-stage respiratory diseases awaiting lung transplantation and in postoperative lung transplant recipients. Most transplant centers recognize the value of assessing frailty in the evaluation of potential candidates for lung transplantation. Frailty has been shown to impact mortality on the waitlist and in the post-transplant period. However, the most effective methods for measuring frailty in lung transplant candidates and recipients have yet to be determined. Strategies to reverse frailty are available and show promising results on outcomes.
Full article
(This article belongs to the Section Solid Organ Transplantation)
►▼
Show Figures
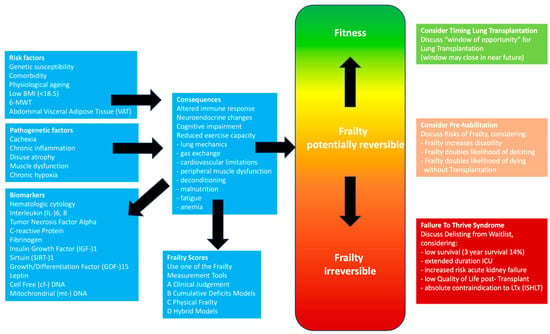
Figure 1
Open AccessArticle
The Impact of Quadriceps Muscle Layer Thickness on Length of Stay of Patients Listed for Renal Transplant
by
Max Levine, Patrick Luke, Alp Sener, Heather Resvick, Stephanie Braga, Taralynn St. Kitts, Sarah De Marinis and Janet Madill
Transplantology 2023, 4(4), 230-241; https://doi.org/10.3390/transplantology4040022 - 13 Dec 2023
Abstract
Background: Quadriceps muscle layer thickness (QMLT), which is measured using ultrasound, is an emerging strategy to identify sarcopenia. Purpose: The purpose of the study was to assess whether pre-operative QMLT values are associated with a prolonged length of stay (LOS; defined as >14
[...] Read more.
Background: Quadriceps muscle layer thickness (QMLT), which is measured using ultrasound, is an emerging strategy to identify sarcopenia. Purpose: The purpose of the study was to assess whether pre-operative QMLT values are associated with a prolonged length of stay (LOS; defined as >14 days) following a renal transplant. Methods: Between March 2019 and January 2020, we performed a prospective study among patients undergoing renal transplantation. Physical Frailty scores and QMLT measurements were performed pre-operatively. The primary outcome was a greater LOS following transplant. Secondary outcomes included complications and renal function. Statistical analysis: Percentiles divided patients into two categories of QMLT (low and high). Continuous outcomes were compared using a two-sided t-test or Mann–Whitney U test, and Chi-square analysis and Fisher exact testing were used for nominal variables. Results: Of 79 patients, the frailty prevalence was 16%. Among patients with low and higher QMLTs, LOS of >14 days were 21% vs. 3% [p = 0.04], respectively. Demographically, there was a higher percentage of patients with living donors in the high- vs. low-QMLT groups (40 vs. 7%). However, in a subgroup analysis excluding living-donor recipients, the difference between groups was preserved (23% vs. 0%, p = 0.01). No differences in secondary outcomes were seen between groups. Conclusions: Low quadriceps muscle layer thickness may be associated with a prolonged length of stay for renal recipients. Further research is needed to confirm our findings.
Full article
(This article belongs to the Section Solid Organ Transplantation)
Open AccessArticle
Nutritional and Sarcopenia Assessment in Bilateral Lung Transplantation Recipient: Can “The Strongest One” Expect Improved Short-Term Outcomes?
by
Sabrina Congedi, Annalisa Boscolo, Marco Nardelli, Martina Biscaro, Christian Legnaro, Nicolò Sella, Giulia Fichera, Tommaso Antonio Giacon, Paola Zanon, Davide Lovison, Mara Bassi, Bianca Maria Borrelli, Giulia Lorenzoni, Chiara Giraudo, Dario Gregori, Federico Rea and Paolo Navalesi
Transplantology 2023, 4(4), 218-229; https://doi.org/10.3390/transplantology4040021 - 16 Nov 2023
Abstract
►▼
Show Figures
Background: Scant data are available on nutritional status in bilateral lung transplant (BLT) candidates. Methods: All consecutive recipients admitted to the intensive care unit (ICU) of the University Hospital of Padua (February 2016–2020) after bilateral-lung transplant (BLT) were retrospectively screened. Data collected: (i)
[...] Read more.
Background: Scant data are available on nutritional status in bilateral lung transplant (BLT) candidates. Methods: All consecutive recipients admitted to the intensive care unit (ICU) of the University Hospital of Padua (February 2016–2020) after bilateral-lung transplant (BLT) were retrospectively screened. Data collected: (i) nutritional indices (body mass index (BMI), albumin level, prognostic nutritional index (PNI), mini nutritional assessment short-form (MNA-SF)); and (ii) muscular indices (creatinine height index (CHI)), skeletal muscle index (SMI), densitometry of paravertebral muscles on chest CT). Results: 108 BLT recipients were enrolled: 55% had a normal BMI, 83% had serum albumin levels > 35 g/L; high PNI and MNA-SF scores were recorded in most of patients. A total of 74% had a “normal or slightly reduced protein state“ according to the CHI score; 17% were identified as “sarcopenic” according to muscle densitometry (Hu < 30). Lower serum albumin was associated with longer invasive mechanical ventilation days (IMV) and ICU length of stay (p-value for non-linearity < 0.01). PNI and BMI were also associated with an increased ICU length of stay (p-value for non-linearity < 0.01). Conclusions: Most of the BLT recipients had normal nutritional and sarcopenia status. Pre-transplant albumin values correlated with the duration of IMV; serum albumin, PNI and BMI were associated with ICU stay. No nutritional or muscle parameters predicted re-intubation, 30-days rejection and overall length of hospital stay.
Full article
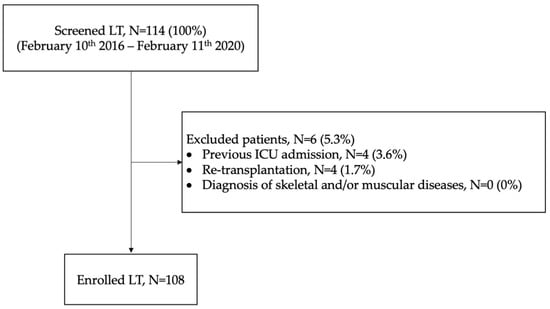
Figure 1
Open AccessArticle
Burden of Renal Dysfunction and Neurologic Complications in Hospitalized Pediatric Heart Failure Unrelated to Congenital Heart Disease: A Multicenter Study
by
Bibhuti Das, Justin Godown and Shriprasad R. Deshpande
Transplantology 2023, 4(4), 209-217; https://doi.org/10.3390/transplantology4040020 - 14 Nov 2023
Abstract
Objectives: Limited data are available on renal dysfunction and neurologic complications in heart failure in children, when the heart failure is not related to congenital heart disease (CHD) or cardiac surgery. This study used a multi-center database to describe pediatric heart failure (pHF)-related
[...] Read more.
Objectives: Limited data are available on renal dysfunction and neurologic complications in heart failure in children, when the heart failure is not related to congenital heart disease (CHD) or cardiac surgery. This study used a multi-center database to describe pediatric heart failure (pHF)-related renal dysfunction, neurological complications, and outcomes in non-CHD patients. Methods: The Pediatric Health Information System (PHIS) database between 2004 and 2020 was used to analyze the prevalence of renal dysfunction and neurologic complications associated with pHF hospitalizations and their impact on outcomes. Results: Of the 5515 hospitalizations included in the study, renal dysfunction was identified in 1239 (22.5%), and neurologic dysfunction was diagnosed in 539 (9.8%). The diagnosis of renal or neurologic complications was associated with significantly higher use of ICU therapies, including mechanical ventilation, parenteral nutrition, and mechanical circulatory support. Patients with significant renal dysfunction were likely to receive kidney transplants in 3.1% of the cases. Neurologic complications were higher in patients with pHF who underwent heart transplantation (21.3% vs. 7.8%, p < 0.001). Patients with renal dysfunction and neurologic complications had significantly higher mortality rates than those without renal dysfunction (11.7% vs. 4.3%, p < 0.001) and neurologic complications (18.4% vs. 4.6%, p < 0.001). Conclusions: Renal dysfunction and neurologic complications are common, resulting in significantly higher utilization of ICU therapies and mortality rates during non-CHD-related pHF hospitalization. Neurologic complications associated with hospitalization for pHF are associated with a significantly higher mortality, which has been underemphasized in the literature. This study assesses the burden of these morbidities and highlights the importance of monitoring and managing renal and neurologic complications in pHF to improve outcomes.
Full article
(This article belongs to the Section Pediatric Transplantation)
Open AccessArticle
Long Survival Following Lung Transplantation: What Matters?
by
Jane Y. Zhao, Doug A. Gouchoe, William E. Schwartzman, Justin P. Rosenheck, Victor Heh, Matthew C. Henn, Nahush A. Mokadam, David R. Nunley, Bryan A. Whitson and Asvin M. Ganapathi
Transplantology 2023, 4(4), 197-208; https://doi.org/10.3390/transplantology4040019 - 31 Oct 2023
Abstract
A retrospective review of the UNOS/OPTN Database was performed from 1 October 1987–31 December 2019. Recipients were classified as LSu (15+ years survival without GF/ReTx), normal survival (3–15 years) and short survival (<3 years). In total, 22,646 patients were identified. Groups were assessed
[...] Read more.
A retrospective review of the UNOS/OPTN Database was performed from 1 October 1987–31 December 2019. Recipients were classified as LSu (15+ years survival without GF/ReTx), normal survival (3–15 years) and short survival (<3 years). In total, 22,646 patients were identified. Groups were assessed with comparative statistics in addition to a multivariate analysis which included recipient, donor, transplant characteristics and select post-transplant complications. LSu recipients were younger, more commonly female, healthier and more commonly had cystic fibrosis, pulmonary vascular disease or bilateral lung transplantation. LSu donors were younger, healthier and lacked clinical infection. Recipients with restrictive lung disease, single lung transplant and dialysis postoperatively were less likely to be LSu. Several recipient, donor and transplant characteristics are associated with long lung transplantation survival. While some factors cannot be altered, others related to donor selection and posttransplant management can potentially be influenced. Understanding these characteristics and employing discretion in donor selection, in appropriate recipients, may optimize the longevity of transplanted lungs.
Full article
(This article belongs to the Section Solid Organ Transplantation)
►▼
Show Figures
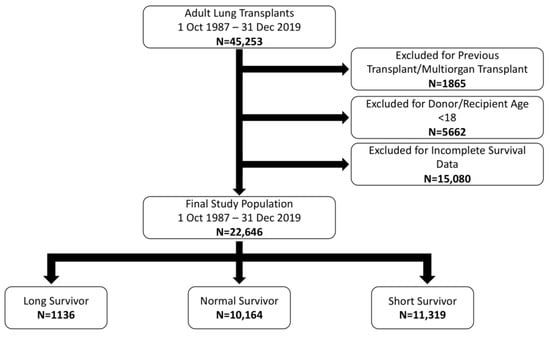
Figure 1
Open AccessArticle
Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model
by
Carmen A. Moes, C. Tji Gan, Leonie H. Venema, Roland F. Hoffmann, Barbro N. Melgert, Huib A. M. Kerstjens, Peter Olinga and Mitchel J. R. Ruigrok
Transplantology 2023, 4(4), 185-196; https://doi.org/10.3390/transplantology4040018 - 25 Oct 2023
Abstract
►▼
Show Figures
Background: Donor lungs are often discarded, with gastric aspiration accounting for ~9% of lungs unsuitable for transplantation. To increase the donor pool, it is important to understand the pathophysiology of aspiration-induced lung damage (AILD) and to assess its treatment. Methods: Precision-cut
[...] Read more.
Background: Donor lungs are often discarded, with gastric aspiration accounting for ~9% of lungs unsuitable for transplantation. To increase the donor pool, it is important to understand the pathophysiology of aspiration-induced lung damage (AILD) and to assess its treatment. Methods: Precision-cut lung slices (PCLS) were prepared from murine lungs and exposed to acid—pH 1.5 to 5.5—for 15 min. We also investigated whether acid-exposed slices (pH 3.5) could affect unexposed slices. In addition, we investigated whether dexamethasone (0.5 or 1 μM) could mitigate and treat the damage in each group. In each experiment (n = 3), we analyzed cell viability (ATP/protein content) and markers of inflammation (IL-1β, IL-6, TNF-𝛼, TRAIL). Results: PCLS subjected to pH 1.5–3.5 had a significantly reduced amount of ATP, albeit no increase in inflammation markers. There was no interaction of secretions from acid-exposed slices on unexposed slices. Dexamethasone had no beneficial effects in either group. Conclusion: Direct exposure to acid in the PCLS leads to a decrease in cell viability. Acid-exposed slices had no effect on the cell viability of unexposed slices. Treatment with dexamethasone offered no mitigation. More studies have to be performed to elucidate the pathophysiology of AILD and the possible treatment of aspiration-induced injury.
Full article
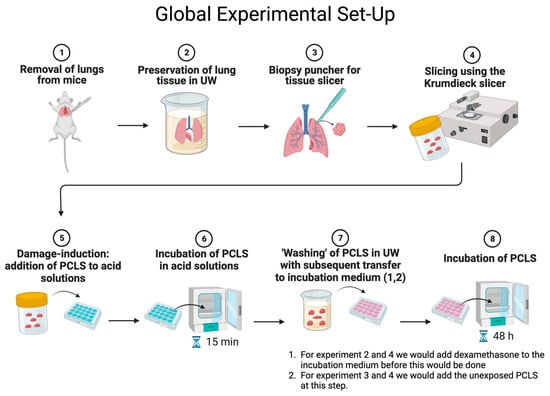
Figure 1
Open AccessCase Report
Diagnostic Challenge in Renal Transplantation: Splenosis vs. Post-Transplant Lymphoproliferative Disorder—A Case Report
by
Jes M. Sanders, Daniel Galvez, Xiaoqi Lin and Joseph Leventhal
Transplantology 2023, 4(3), 178-184; https://doi.org/10.3390/transplantology4030017 - 21 Sep 2023
Abstract
Splenosis is a benign, acquired condition characterized by the auto-implantation of focal deposits of splenic tissue throughout the peritoneal cavity, most commonly occurring after splenic injury and/or splenectomy. Post-Transplant Lymphoproliferative Disorder (PTLD) is a well-known complication of solid organ transplantation that results from
[...] Read more.
Splenosis is a benign, acquired condition characterized by the auto-implantation of focal deposits of splenic tissue throughout the peritoneal cavity, most commonly occurring after splenic injury and/or splenectomy. Post-Transplant Lymphoproliferative Disorder (PTLD) is a well-known complication of solid organ transplantation that results from unregulated B-cell proliferation due to chronic immunosuppression. Given their clinical and radiologic similarities, these two entities may pose a diagnostic dilemma in select solid-organ transplant recipients. We present the case of a 54-year-old kidney-transplant recipient presenting with abdominal pain and found to have a retroperitoneal soft-tissue mass concerning for PTLD. He underwent a CT-guided biopsy of the mass, and histopathological studies revealed lymphoid tissue consistent with splenic tissue, thus ruling out PTLD. The patient subsequently underwent symptomatic management, with the eventual resolution of his symptoms. The early diagnosis of PTLD is paramount, as prompt intervention has a substantial impact on the high rate of morbidity and mortality associated with this condition. Additionally, the diagnosis of splenosis in the setting of a retroperitoneal mass is critical in order to avoid invasive diagnostic and therapeutic procedures that may result in significant complications. A detailed surgical history, including prior splenic trauma and/or splenectomy, should raise clinical suspicion for splenosis and guide further diagnostic and therapeutic decision making.
Full article
(This article belongs to the Section Special Clinical Cases and Videos)
►▼
Show Figures
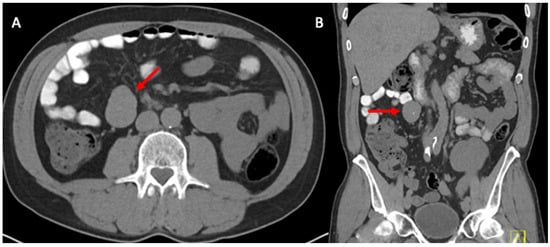
Figure 1
Open AccessReview
Recurrent Immunoglobulin A Nephropathy after Kidney Transplant—An Updated Review
by
Hwarang S. Han, Michelle L. Lubetzky, Nidharshan S. Anandasivam, Rebecca A. Cox and Brian K. Lee
Transplantology 2023, 4(3), 161-177; https://doi.org/10.3390/transplantology4030016 - 06 Sep 2023
Abstract
Immunoglobulin A nephropathy (IgAN) is the commonest glomerulonephritis worldwide, a category that represents the third most frequent cause of end-stage kidney disease (ESKD) in the United States. Kidney transplantation remains the optimal treatment of ESKD, and yet the prospects of IgAN recurrence post-transplant
[...] Read more.
Immunoglobulin A nephropathy (IgAN) is the commonest glomerulonephritis worldwide, a category that represents the third most frequent cause of end-stage kidney disease (ESKD) in the United States. Kidney transplantation remains the optimal treatment of ESKD, and yet the prospects of IgAN recurrence post-transplant dampens the enthusiasm for living kidney donation in some instances, in addition to limiting the longevity of the kidney allograft. Moreover, the lack of a standardized method for detecting IgAN recurrence, since not all centers perform protocol allograft biopsies, has led to an underestimation of the extent of the issue. The pathogenesis of de novo IgAN remains conjectural, let alone the pathways for recurrent disease, but is increasingly recognized as a multi-hit injury mechanism. Identification of recurrent disease rests mainly on clinical symptoms and signs (e.g., hematuria, proteinuria) and could only be definitively proven with histologic evidence which is invasive and prone to sampling error. Treatment had relied mainly on nonspecific goals of proteinuria reduction, and in some cases, immunosuppression for active, crescentic disease. More recently, newer targets have the potential to widen the armamentarium for directed therapies, with more studies on the horizon. This review article provides an update on recurrent IgAN post-transplant.
Full article
(This article belongs to the Special Issue Challenges and Opportunities of Kidney Transplantation in Patients with Immune Diseases)
Open AccessCase Report
Upfront Normothermic Machine Perfusion for a Liver Graft with Severe Macrovesicular Steatosis: A Proof-of-Concept Case
by
Damiano Patrono, Ana Lavinia Apostu, Giorgia Rizza, Davide Cussa, Antonella Barreca, Selene Limoncelli, Stefano Mirabella and Renato Romagnoli
Transplantology 2023, 4(3), 151-160; https://doi.org/10.3390/transplantology4030015 - 23 Aug 2023
Abstract
Graft steatosis has been associated with inferior outcomes after liver transplantation. Given the rising prevalence of obesity and fatty liver disease, strategies allowing safe and successful utilization of fatty liver grafts are needed. Liver preservation by normothermic machine perfusion (NMP) allows reducing ischemia-reperfusion
[...] Read more.
Graft steatosis has been associated with inferior outcomes after liver transplantation. Given the rising prevalence of obesity and fatty liver disease, strategies allowing safe and successful utilization of fatty liver grafts are needed. Liver preservation by normothermic machine perfusion (NMP) allows reducing ischemia-reperfusion injury, extending preservation time and assessing graft viability prior to implantation into the recipient. NMP can be initiated at the donor hospital using a transportable device (referred to as upfront NMP or normothermic machine preservation) or after a period of cold ischemia (known as back-to-base). In this report, we present the case of a graft from an HCV-positive DBD donor with 70% macrovesicular steatosis, which was successfully preserved and transplanted using upfront NMP. This approach was key to minimize initial injury to the graft and allowed assessing its viability before transplantation, while improving transplant logistics. Upfront NMP represents a promising approach to enhance the transplantation of fatty liver grafts.
Full article
(This article belongs to the Section Organ and Tissue Donation and Preservation)
►▼
Show Figures
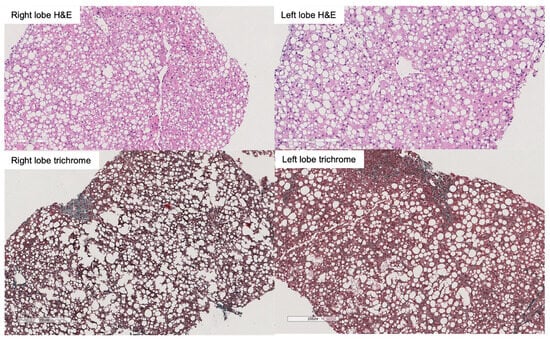
Figure 1
Open AccessReview
Update on Desensitization Strategies and Drugs on Hyperimmune Patients for Kidney Transplantation
by
Maurizio Salvadori
Transplantology 2023, 4(3), 139-150; https://doi.org/10.3390/transplantology4030014 - 08 Aug 2023
Cited by 1
Abstract
The presence in a recipient of antibodies directed against donor-specific antigens represents a major obstacle to transplantation. Removal of these antibodies represents a challenge for physicians dealing with kidney transplantation. Several strategies, techniques, and old and new drugs are currently used for desensitizing
[...] Read more.
The presence in a recipient of antibodies directed against donor-specific antigens represents a major obstacle to transplantation. Removal of these antibodies represents a challenge for physicians dealing with kidney transplantation. Several strategies, techniques, and old and new drugs are currently used for desensitizing these patients. Desensitization may either occur before transplantation, at the time of transplantation, or after transplantation according to whether physicians are dealing with living or deceased donors. Different techniques may be used to reveal the presence of antibodies in the recipients; each technique has different sensitivities and specificities, and different advantages and drawbacks. The targets of the drugs used to desensitize are B cells, plasma cells, the antibodies themselves, and, finally, the complement that is the final actor causing tissue disruption. B cells are relatively easy to target; targeting the plasma cell is more difficult. Indeed, several new drugs are also used in randomized trials to defeat plasma cells. Antibodies may be removed easily, but their removal is often followed by antibody rebound. The complement is not easy to defeat and new drugs are currently used for this aim. Overall, despite difficulties, desensitization is currently possible in many cases, to obtain a safe and successful transplantation.
Full article
(This article belongs to the Special Issue Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients)
►▼
Show Figures

Figure 1
Open AccessReview
Mechanisms of Cold Preservation and Reperfusion Injury for Solid Organ Transplantation: Implications for Partial Heart Transplantations
by
Corey Mealer, Haley Konsek, Zachary Travis, Rebecca N. Suk and Taufiek Konrad Rajab
Transplantology 2023, 4(3), 124-138; https://doi.org/10.3390/transplantology4030013 - 18 Jul 2023
Abstract
►▼
Show Figures
Cold preservation is a key component to organ procurement and transplantation. Cold preservation functions by slowing metabolic activity of procured organs and begins the period known as cold ischemic time (CIT). Reducing CIT and warm ischemic time (WIT) are paramount to minimizing donor
[...] Read more.
Cold preservation is a key component to organ procurement and transplantation. Cold preservation functions by slowing metabolic activity of procured organs and begins the period known as cold ischemic time (CIT). Reducing CIT and warm ischemic time (WIT) are paramount to minimizing donor organ damage from ischemia and the build-up of waste products and signals that drive reperfusion injury prior to transplantation into a matching recipient. Preventing damage from CIT and WIT and extending the amount of time that organs can tolerate has been a major goal of organ transplantation since donors and recipients are frequently not located within the same hospital, region, or state. Meanwhile, the amount of CIT that a transplant center is willing to accept differs based on the organ, the institution receiving the organ offer, and the doctor receiving the offer for that institution. With the introduction of a partial heart transplantation conducted last year at Duke University, it is important to discuss how much CIT transplant centers conducting a partial heart transplantation (pHT) are willing to accept. This article will review the physiology of WIT and CIT, associated organ damage, CIT variation among transplant centers and organ types, and provide a brief discussion of the future of pHT-accepted CIT and the need for research in this field.
Full article
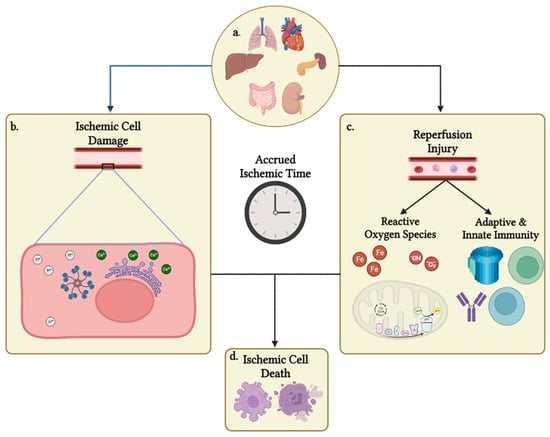
Figure 1
Open AccessArticle
Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study
by
Luis Carlos Alvarez-Perdomo, John Ubeimar Cataño-Bedoya, Maribel Plaza-Tenorio, Ana María Botero-Mora, Isabel del Pilar Cardozo-Moreno, Luis Manuel Barrera-Lozano, Jaime Alberto Ramírez-Arbeláez and Carlos M. Ardila
Transplantology 2023, 4(3), 111-123; https://doi.org/10.3390/transplantology4030012 - 14 Jul 2023
Abstract
The purpose of the study was to characterize lower extremity peripheral arterial disease (LEPAD) in a series of kidney transplant patients and to assess the impact on adverse outcomes. A retrospective cohort study was conducted including kidney transplant recipient patients who underwent screening
[...] Read more.
The purpose of the study was to characterize lower extremity peripheral arterial disease (LEPAD) in a series of kidney transplant patients and to assess the impact on adverse outcomes. A retrospective cohort study was conducted including kidney transplant recipient patients who underwent screening for LEPAD. The outcomes evaluated were classified as perioperative and post-transplant, including cardiovascular events, amputation, mortality, and loss of the graft. A total of 141 renal transplant patients screened for LEPAD were identified, with an average follow-up of 3 years. LEPAD occurred in 14.2% (20/141). No differences in cardiovascular risk factors were found between the groups, except for smoking (45% vs. 24%, p < 0.05). In the group with LEPAD, the most compromised anatomical segment was the infrapopliteus, with no iliac involvement found. The Cox proportional hazards model indicated that the variables age, gender, and weight were significant in patients with LEPAD. There were no differences between the groups in terms of graft loss and death. The infrapopliteal segment is the area of greatest stenosis in kidney transplant patients with LEPAD. Together with smoking, they can explain the presence of major amputations in kidney transplant patients; however, they had no impact on graft functionality or death.
Full article
(This article belongs to the Special Issue Recent Advances in Dialysis and Kidney Transplantation)
►▼
Show Figures
Figure 1
Open AccessArticle
A Combination of Cytological Biomarkers as a Guide in the Diagnosis of Acute Rejection in Lung Transplant Recipients
by
Silvia Aguado Ibáñez, Rosalía Laporta Hernández, Myriam Aguilar Pérez, Christian García Fadul, Cristina López García Gallo, Gema Díaz Nuevo, Sonia Salinas Castillo, Raquel Castejón Diaz, Clara Salas Anton, Ana Royuela Vicente, Francisco Antonio Bernabeu Andreu and María Piedad Ussetti Gil
Transplantology 2023, 4(3), 102-110; https://doi.org/10.3390/transplantology4030011 - 25 Jun 2023
Abstract
►▼
Show Figures
The usefulness of bronchoalveolar lavage fluid (BALF) to support the diagnosis of acute cellular (ACR) rejection in lung transplant (LTX) recipients remains controversial. ACR has been associated with blood eosinophil counts (EOS) in other solid organ recipients, but there are few studies in
[...] Read more.
The usefulness of bronchoalveolar lavage fluid (BALF) to support the diagnosis of acute cellular (ACR) rejection in lung transplant (LTX) recipients remains controversial. ACR has been associated with blood eosinophil counts (EOS) in other solid organ recipients, but there are few studies in relation to lung transplants. Our aim was to assess the usefulness of the combined analysis of BALF cellularity and EOS for the diagnosis of ACR in lung transplant recipients. This is a retrospective study of findings observed simultaneously in 887 transbronchial biopsies (TBB), BALF, and blood samples obtained from 363 LTx patients transplanted between 2014 and 2020. The variables collected were: demographics, ACR degree, BALF cellularity, and simultaneous blood EOS counts. The lymphocyte count in BALF was significantly higher in patients with ACR than in those without (11.35% vs. 6.11%; p < 0.001). In parallel, EOS counts were also significantly higher in patients with ACR than in the non-ACR group (EOS 213 ± 206/mm3 vs. 83 ± 129/mm3; p < 0.001). Increases in both parameters were associated with an increased risk of ACR (lymphocytes OR 1.100; 95% CI 1.080–1.131; EOS OR 1.460; 95% CI 1.350–1.580). The diagnostic specificity of ACR for a lymphocyte count > 12% was 71.1%, which increased to 95.8% when taking into account a simultaneous blood EOS count > 200/mm3. Simultaneous assessment of BALF lymphocyte counts and blood eosinophil counts may be useful for diagnosing ACR in patients with risk factors for TBB or in the presence of inconclusive histological samples.
Full article
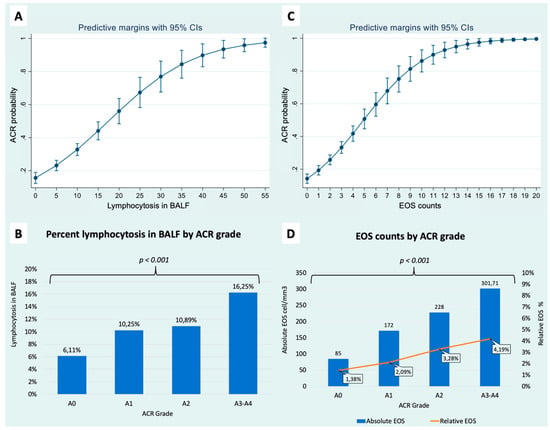
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
Transplantology
Strategies for Access to Kidney Transplantation for Highly Sensitized and Incompatible Patients
Guest Editor: Maurizio SalvadoriDeadline: 31 December 2024
Topical Collections
Topical Collection in
Transplantology
Progress and Recent Advances in Solid Organ Transplantation
Collection Editors: Charat Thongprayoon, Wisit Cheungpasitporn, Wisit Kaewput



