Immunotoxins 2016
A topical collection in Toxins (ISSN 2072-6651).
Viewed by 73674
Share This Topical Collection
Editors
 Dr. David J. Fitzgerald
Dr. David J. Fitzgerald
 Dr. David J. Fitzgerald
Dr. David J. Fitzgerald
E-Mail
Collection Editor
Laboratory of Molecular Biology, CCR, NCI, NIH, HHS, 37 Convent Dr, Room 5124, Bethesda, MD, 20892, USA
Interests: antibodies; toxins; cancer therapeutics; cell biology; immunotoxins
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Immunotoxins (IT) constitute a group of biochemical constructs that target cells with precise specificity. The rationale of IT development was based on the concept of the “magic bullet”, devised by Paul Ehrlich in the late 19th and early 20th century. ITs are composed of a carrier domain, which is able to identify and bind a cell target, and a toxic domain, which kills the cell target. ITs may be constructed using conventional chemical cross-linkers or joined via recombinant DNA technology. When constructing conventional ITs, domains are purified separately and then attached to one another with chemical linkers. In making recombinant ITs (rIT), domains are fused through gene tailoring and then expressed as a single chimeric protein. Carrier domains are usually composed of antibodies or antibody-derived fragments. Toxic domains are typically from either intact plants or they are derived from microbial toxins. Modified toxins are engineered to retain an active species, often an enzyme, with reduced unspecific toxicity. ITs have been targeted to treat a number of diseases, but mainly cancer. Understanding the mechanisms involved in IT toxicity and specificity for target cells, and overall safety are very important issues. To improve clinical outcomes, immunotoxins are being combined with so-called enhancer compounds.
Prof. Tomas Girbes
Dr. David J. Fitzgerald
Collection Editors
Submission
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be published continuously (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed Open Access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1400 CHF (Swiss Francs).
Keywords
- conventional immunotoxins
- recombinant immunotoxins
- mechanism of action of immunotoxins
- enhancers of immunotoxin toxicity
- targeted therapeutics
Published Papers (9 papers)
2016
Open AccessArticle
Towards Engineering Novel PE-Based Immunotoxins by Targeting Them to the Nucleus
by
Marta Borowiec, Michal Gorzkiewicz, Joanna Grzesik, Aurelia Walczak-Drzewiecka, Anna Salkowska, Ewelina Rodakowska, Kamil Steczkiewicz, Leszek Rychlewski, Jaroslaw Dastych and Krzysztof Ginalski
Cited by 13 | Viewed by 6191
Abstract
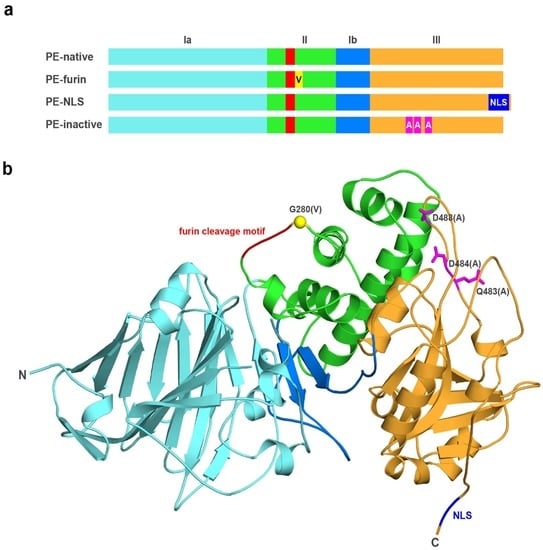
Exotoxin A (PE) from
Pseudomonas aeruginosa is a bacterial ADP-ribosyltransferase, which can permanently inhibit translation in the attacked cells. Consequently, this toxin is frequently used in immunotoxins for targeted cancer therapies. In this study, we propose a novel modification to PE by incorporating
[...] Read more.
Exotoxin A (PE) from
Pseudomonas aeruginosa is a bacterial ADP-ribosyltransferase, which can permanently inhibit translation in the attacked cells. Consequently, this toxin is frequently used in immunotoxins for targeted cancer therapies. In this study, we propose a novel modification to PE by incorporating the NLS sequence at its
C-terminus, to make it a selective agent against fast-proliferating cancer cells, as a nucleus-accumulated toxin should be separated from its natural substrate (eEF2) in slowly dividing cells. Here, we report the cytotoxic activity and selected biochemical properties of newly designed PE mutein using two cellular models: A549 and HepG2. We also present a newly developed protocol for efficient purification of recombinant PE and its muteins with very high purity and activity. We found that furin cleavage is not critical for the activity of PE in the analyzed cell lines. Surprisingly, we observed increased toxicity of the toxin accumulated in the nucleus. This might be explained by unexpected nuclease activity of PE and its potential ability to cleave chromosomal DNA, which seems to be a putative alternative intoxication mechanism. Further experimental investigations should address this newly detected activity to identify catalytic residues and elucidate the molecular mechanism responsible for this action.
Full article
►▼
Show Figures
Open AccessReview
Glypican-3 Targeting Immunotoxins for the Treatment of Liver Cancer
by
Bryan D. Fleming and Mitchell Ho
Cited by 21 | Viewed by 7629
Abstract
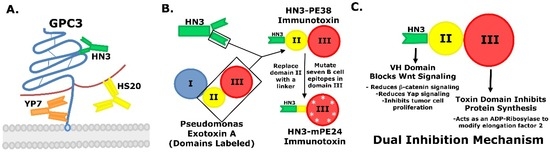
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, yet no effective therapeutics exist. This review provides an overview of the recent development of recombinant immunotoxins for the treatment of glypican-3 (GPC3) expressing HCC. GPC3 is a cell surface heparan
[...] Read more.
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, yet no effective therapeutics exist. This review provides an overview of the recent development of recombinant immunotoxins for the treatment of glypican-3 (GPC3) expressing HCC. GPC3 is a cell surface heparan sulfate proteoglycan that is overexpressed in HCC, but is absent from normal adult human tissues. Treatment of HCC with anti-GPC3 immunotoxins represents a new therapeutic option. Using phage display and hybridoma technologies, three high affinity antibodies (HN3, HS20 and YP7) have been generated against GPC3. Two of these antibodies (HN3 and HS20) have demonstrated the ability to inhibit Wnt/Yap signaling, leading to a reduction in liver cancer cell proliferation. By combining the HN3 antibody capable of inhibiting Wnt/Yap signaling with the protein synthesis inhibitory domain of the Pseudomonas exotoxin, a recombinant immunotoxin that exhibits a dual inhibitory mechanism was generated. This immunotoxin was found to be highly effective in the treatment of human HCCs in mouse xenograft models. Engineering of the toxin fragment to reduce the level of immunogenicity is currently being explored. The development of immunotoxins provides opportunities for novel liver cancer therapies.
Full article
►▼
Show Figures
Open AccessArticle
Protection of the Furin Cleavage Site in Low-Toxicity Immunotoxins Based on Pseudomonas Exotoxin A
by
Gilad Kaplan, Fred Lee, Masanori Onda, Emily Kolyvas, Gaurav Bhardwaj, David Baker and Ira Pastan
Cited by 24 | Viewed by 6459
Abstract
Recombinant immunotoxins (RITs) are fusions of an Fv-based targeting moiety and a toxin.
Pseudomonas exotoxin A (PE) has been used to make several immunotoxins that have been evaluated in clinical trials. Immunogenicity of the bacterial toxin and off-target toxicity have limited the efficacy
[...] Read more.
Recombinant immunotoxins (RITs) are fusions of an Fv-based targeting moiety and a toxin.
Pseudomonas exotoxin A (PE) has been used to make several immunotoxins that have been evaluated in clinical trials. Immunogenicity of the bacterial toxin and off-target toxicity have limited the efficacy of these immunotoxins. To address these issues, we have previously made RITs in which the Fv is connected to domain III (PE24) by a furin cleavage site (FCS), thereby removing unneeded sequences of domain II. However, the PE24 containing RITs do not contain the naturally occurring disulfide bond around the furin cleavage sequence, because it was removed when domain II was deleted. This could potentially allow PE24 containing immunotoxins to be cleaved and inactivated before internalization by cell surface furin or other proteases in the blood stream or tumor microenvironment. Here, we describe five new RITs in which a disulfide bond is engineered to protect the FCS. The most active of these, SS1-Fab-DS3-PE24, shows a longer serum half-life than an RIT without the disulfide bond and has the same anti-tumor activity, despite being less cytotoxic in vitro. These results have significance for the production of de-immunized, low toxicity, PE24-based immunotoxins with a longer serum half-life.
Full article
►▼
Show Figures
Open AccessReview
Augmenting the Efficacy of Immunotoxins and Other Targeted Protein Toxins by Endosomal Escape Enhancers
by
Hendrik Fuchs, Alexander Weng and Roger Gilabert-Oriol
Cited by 31 | Viewed by 9577
Abstract
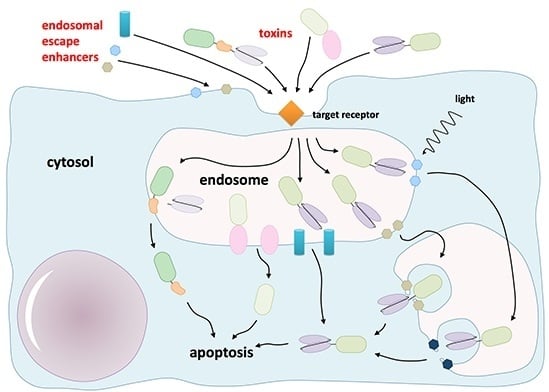
The toxic moiety of almost all protein-based targeted toxins must enter the cytosol of the target cell to mediate its fatal effect. Although more than 500 targeted toxins have been investigated in the past decades, no antibody-targeted protein toxin has been approved for
[...] Read more.
The toxic moiety of almost all protein-based targeted toxins must enter the cytosol of the target cell to mediate its fatal effect. Although more than 500 targeted toxins have been investigated in the past decades, no antibody-targeted protein toxin has been approved for tumor therapeutic applications by the authorities to date. Missing efficacy can be attributed in many cases to insufficient endosomal escape and therefore subsequent lysosomal degradation of the endocytosed toxins. To overcome this drawback, many strategies have been described to weaken the membrane integrity of endosomes. This comprises the use of lysosomotropic amines, carboxylic ionophores, calcium channel antagonists, various cell-penetrating peptides of viral, bacterial, plant, animal, human and synthetic origin, other organic molecules and light-induced techniques. Although the efficacy of the targeted toxins was typically augmented in cell culture hundred or thousand fold, in exceptional cases more than million fold, the combination of several substances harbors new problems including additional side effects, loss of target specificity, difficulties to determine the therapeutic window and cell type-dependent variations. This review critically scrutinizes the chances and challenges of endosomal escape enhancers and their potential role in future developments.
Full article
►▼
Show Figures
Open AccessReview
Tumor Targeting and Drug Delivery by Anthrax Toxin
by
Christopher Bachran and Stephen H. Leppla
Cited by 44 | Viewed by 13152
Abstract
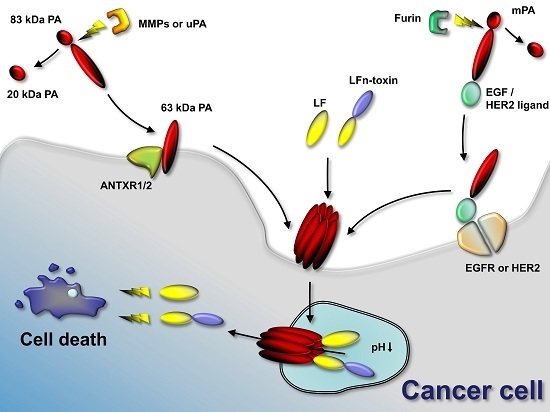
Anthrax toxin is a potent tripartite protein toxin from
Bacillus anthracis. It is one of the two virulence factors and causes the disease anthrax. The receptor-binding component of the toxin, protective antigen, needs to be cleaved by furin-like proteases to be activated
[...] Read more.
Anthrax toxin is a potent tripartite protein toxin from
Bacillus anthracis. It is one of the two virulence factors and causes the disease anthrax. The receptor-binding component of the toxin, protective antigen, needs to be cleaved by furin-like proteases to be activated and to deliver the enzymatic moieties lethal factor and edema factor to the cytosol of cells. Alteration of the protease cleavage site allows the activation of the toxin selectively in response to the presence of tumor-associated proteases. This initial idea of re-targeting anthrax toxin to tumor cells was further elaborated in recent years and resulted in the design of many modifications of anthrax toxin, which resulted in successful tumor therapy in animal models. These modifications include the combination of different toxin variants that require activation by two different tumor-associated proteases for increased specificity of toxin activation. The anthrax toxin system has proved to be a versatile system for drug delivery of several enzymatic moieties into cells. This highly efficient delivery system has recently been further modified by introducing ubiquitin as a cytosolic cleavage site into lethal factor fusion proteins. This review article describes the latest developments in this field of tumor targeting and drug delivery.
Full article
►▼
Show Figures
Open AccessArticle
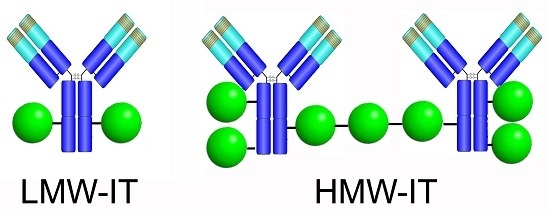
High in Vitro Anti-Tumor Efficacy of Dimeric Rituximab/Saporin-S6 Immunotoxin
by
Massimo Bortolotti, Andrea Bolognesi, Maria Giulia Battelli and Letizia Polito
Cited by 9 | Viewed by 5016
Abstract
The anti-CD20 mAb Rituximab has revolutionized lymphoma therapy, in spite of a number of unresponsive or relapsing patients. Immunotoxins, consisting of toxins coupled to antibodies, are being investigated for their potential ability to augment Rituximab efficacy. Here, we compare the anti-tumor effect of
[...] Read more.
The anti-CD20 mAb Rituximab has revolutionized lymphoma therapy, in spite of a number of unresponsive or relapsing patients. Immunotoxins, consisting of toxins coupled to antibodies, are being investigated for their potential ability to augment Rituximab efficacy. Here, we compare the anti-tumor effect of high- and low-molecular-weight Rituximab/saporin-S6 immunotoxins, named HMW-IT and LMW-IT, respectively. Saporin-S6 is a potent and stable plant enzyme belonging to ribosome-inactivating proteins that causes protein synthesis arrest and consequent cell death. Saporin-S6 was conjugated to Rituximab through an artificial disulfide bond. The inhibitory activity of HMW-IT and LMW-IT was evaluated on cell-free protein synthesis and in two CD20
+ lymphoma cell lines, Raji and D430B. Two different conjugates were separated on the basis of their molecular weight and further characterized. Both HMW-IT (dimeric) and LMW-IT (monomeric) maintained a high level of enzymatic activity in a cell-free system. HMW-IT, thanks to a higher toxin payload and more efficient antigen capping, showed stronger
in vitro anti-tumor efficacy than LMW-IT against lymphoma cells. Dimeric HMW-IT can be used for lymphoma therapy at least for
ex vivo treatments. The possibility of using HMW-IT augments the yield in immunotoxin preparation and allows the targeting of antigens with low internalization rates.
Full article
►▼
Show Figures
Open AccessCommunication
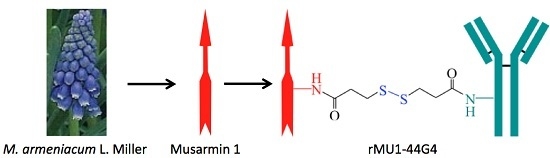
Anti-Human Endoglin (hCD105) Immunotoxin—Containing Recombinant Single Chain Ribosome-Inactivating Protein Musarmin 1
by
Begoña Barriuso, Pilar Antolín, F. Javier Arias, Alessandra Girotti, Pilar Jiménez, Manuel Cordoba-Diaz, Damián Cordoba-Diaz and Tomás Girbés
Cited by 8 | Viewed by 5034
Abstract
Endoglin (CD105) is an accessory component of the TGF-β receptor complex, which is expressed in a number of tissues and over-expressed in the endothelial cells of tumor neovasculature. Targeting endoglin with immunotoxins containing type 2 ribosome-inactivating proteins has proved an effective tool to
[...] Read more.
Endoglin (CD105) is an accessory component of the TGF-β receptor complex, which is expressed in a number of tissues and over-expressed in the endothelial cells of tumor neovasculature. Targeting endoglin with immunotoxins containing type 2 ribosome-inactivating proteins has proved an effective tool to reduce blood supply to B16 mice tumor xenografts. We prepared anti-endoglin immunotoxin (IT)—containing recombinant musarmin 1 (single chain ribosome-inactivating proteins) linked to the mouse anti-human CD105 44G4 mouse monoclonal antibody via
N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP). The immunotoxin specifically killed L929 fibroblast mouse cells transfected with the short form of human endoglin with IC
50 values in the range of 5 × 10
−10 to 10
−9 M.
Full article
►▼
Show Figures
Open AccessReview
CD133, Selectively Targeting the Root of Cancer
by
Jörg U. Schmohl and Daniel A. Vallera
Cited by 74 | Viewed by 10168
Abstract
Cancer stem cells (CSC) are capable of promoting tumor initiation and self-renewal, two important hallmarks of carcinoma formation. This population comprises a small percentage of the tumor mass and is highly resistant to chemotherapy, causing the most difficult problem in the field of
[...] Read more.
Cancer stem cells (CSC) are capable of promoting tumor initiation and self-renewal, two important hallmarks of carcinoma formation. This population comprises a small percentage of the tumor mass and is highly resistant to chemotherapy, causing the most difficult problem in the field of cancer research, drug refractory relapse. Many CSC markers have been reported. One of the most promising and perhaps least ubiquitous is CD133, a membrane-bound pentaspan glycoprotein that is frequently expressed on CSC. There is evidence that directly targeting CD133 with biological drugs might be the most effective way to eliminate CSC. We have investigated two entirely unrelated, but highly effective approaches for selectively targeting CD133. The first involves using a special anti-CD133 single chain variable fragment (scFv) to deliver a catalytic toxin. The second utilizes this same scFv to deliver components of the immune system. In this review, we discuss the development and current status of these CD133 associated biological agents. Together, they show exceptional promise by specific and efficient CSC elimination.
Full article
►▼
Show Figures
Open AccessReview
Immunotoxin Therapies for the Treatment of Epidermal Growth Factor Receptor-Dependent Cancers
by
Nathan Simon and David FitzGerald
Cited by 43 | Viewed by 9148
Abstract
Many epithelial cancers rely on enhanced expression of the epidermal growth factor receptor (EGFR) to drive proliferation and survival pathways. Development of therapeutics to target EGFR signaling has been of high importance, and multiple examples have been approved for human use. However, many
[...] Read more.
Many epithelial cancers rely on enhanced expression of the epidermal growth factor receptor (EGFR) to drive proliferation and survival pathways. Development of therapeutics to target EGFR signaling has been of high importance, and multiple examples have been approved for human use. However, many of the current small molecule or antibody-based therapeutics are of limited effectiveness due to the inevitable development of resistance and toxicity to normal tissues. Recombinant immunotoxins are therapeutic molecules consisting of an antibody or receptor ligand joined to a protein cytotoxin, combining the specific targeting of a cancer-expressed receptor with the potent cell killing of cytotoxic enzymes. Over the decades, many bacterial- or plant-based immunotoxins have been developed with the goal of targeting the broad range of cancers reliant upon EGFR overexpression. Many examples demonstrate excellent anti-cancer properties in preclinical development, and several EGFR-targeted immunotoxins have progressed to human trials. This review summarizes much of the past and current work in the development of immunotoxins for targeting EGFR-driven cancers.
Full article
►▼
Show Figures