Journal Description
Thalassemia Reports
Thalassemia Reports
is an international, peer-reviewed, open access journal on the study, diagnosis, and treatment of thalassemia, published quarterly online by MDPI (from Volume 12 Issue 1-2022).
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Embase, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 23.6 days after submission; acceptance to publication is undertaken in 3.7 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
0.3 (2022)
Latest Articles
Unveiling Extramedullary Hematopoiesis: A Case Report Highlighting the Causes, Symptoms, and Management Strategies
Thalass. Rep. 2024, 14(2), 26-32; https://doi.org/10.3390/thalassrep14020004 - 10 Apr 2024
Abstract
►
Show Figures
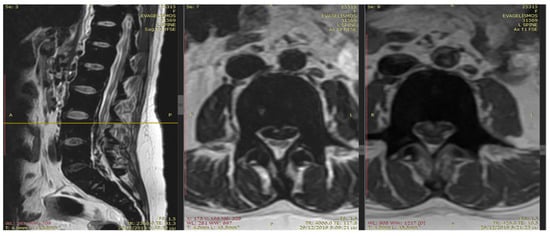
Extramedullary hematopoiesis (EMH) serves as a compensatory mechanism in chronic hemolytic anemias, such as thalassemia, and can result in spinal cord compression. This case report highlights a 36-year-old woman with transfusion-dependent β-thalassemia (TDT) who presented with lower extremity motor deficiency, pelvic paresthesia, and
[...] Read more.
Extramedullary hematopoiesis (EMH) serves as a compensatory mechanism in chronic hemolytic anemias, such as thalassemia, and can result in spinal cord compression. This case report highlights a 36-year-old woman with transfusion-dependent β-thalassemia (TDT) who presented with lower extremity motor deficiency, pelvic paresthesia, and bladder dysfunction. The patient had a history of lower back pain, bilateral lower limb weakness, and demonstrated poor compliance with iron chelation therapy. MRI findings indicated spinal cord compression attributable to extramedullary hematopoiesis. Due to the infeasibility of surgical intervention, the patient underwent hypertransfusion and iron chelation therapy. While neurological symptoms improved, urinary retention persisted. The patient continues to receive iron chelation treatment and undergo transfusions. Managing extramedullary hematopoiesis in thalassemia necessitates an individualized treatment approach.
Full article
Open AccessCase Report
A Case Report of Hyperhemolytic Syndrome in Sickle Cell Disease, with a Special Focus on Avoiding the Use of Transfusions
by
Omar Obajed Al-Ali, György Pfliegler, Ferenc Magyari, Fanni Borics, László Imre Pinczés, Árpád Illés and Boglárka Brúgós
Thalass. Rep. 2024, 14(1), 18-25; https://doi.org/10.3390/thalassrep14010003 - 04 Mar 2024
Abstract
►▼
Show Figures
In patients with sickle cell disease (SCD), transfusions pose risks like delayed hemolytic transfusion reaction (DHTR) and hyperhemolytic syndrome (HHS). We present the case of a 61-year-old Nigerian male patient with SCD, developing hyperhemolytic syndrome (HHS) post-orthopedic surgery due to alloimmunization from blood
[...] Read more.
In patients with sickle cell disease (SCD), transfusions pose risks like delayed hemolytic transfusion reaction (DHTR) and hyperhemolytic syndrome (HHS). We present the case of a 61-year-old Nigerian male patient with SCD, developing hyperhemolytic syndrome (HHS) post-orthopedic surgery due to alloimmunization from blood transfusions. Surgery induced massive hemorrhage, requiring RBC transfusions. Postoperatively, he developed HHS with jaundice, hemoglobinuria, and fever. Despite additional transfusions, his condition worsened, leading to hematological consultation on postoperative day +9. Laboratory findings showed positive DAT and multiple alloantibodies. The diagnosis of HHS was established and treatment involved high-dose methylprednisolone, intravenous immunoglobulin (IVIG), and erythropoietin. The patient was discharged on postoperative day +24 with stable hemoglobin levels, tapering doses of methylprednisolone, and continuous administration of hydroxyurea prescribed. HHS pathogenesis involves extensive intravascular hemolysis, exacerbated by alloimmunization. Diagnostic challenges and therapy selection complexity underscore the need for cautious transfusion strategies in HHS, reserving them for hemodynamic instability or hypoxia. This case highlights promptly recognizing and managing HHS in SCD for improved outcomes and avoiding unnecessary transfusions.
Full article
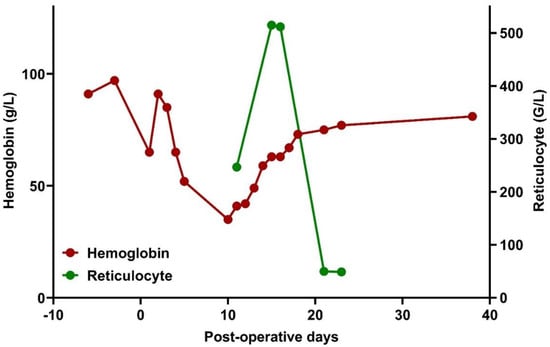
Figure 1
Open AccessArticle
Causes of Hospitalizations in Pediatric Patients with Thalassemia under the National Health Coverage Scheme in Thailand
by
Pimlak Charoenkwan, Patcharee Komvilaisak, Kaewjai Thepsuthummarat, Panya Seksarn and Kitti Torcharus
Thalass. Rep. 2024, 14(1), 10-17; https://doi.org/10.3390/thalassrep14010002 - 01 Mar 2024
Abstract
Thalassemia is a hereditary hemolytic anemia that is prevalent in Southeast Asia. The primary treatment for severe thalassemia involves red cell transfusion, iron chelation, and the treatment of long-term complications, leading to frequent hospital visits and admissions. This study aims to delineate the
[...] Read more.
Thalassemia is a hereditary hemolytic anemia that is prevalent in Southeast Asia. The primary treatment for severe thalassemia involves red cell transfusion, iron chelation, and the treatment of long-term complications, leading to frequent hospital visits and admissions. This study aims to delineate the causes and characteristics of hospital admissions among thalassemia patients under the National Health Coverage (NHC) scheme in Thailand. This cross-sectional analysis (2015–2019), conducted using the National Health Security Office database, identified 336,054 admissions among 41,237 patients, with alpha-thalassemia at 12.5%, beta-thalassemia at 61.5%, other thalassemia at 0.5%, and unclassified thalassemia at 25.5%. The overall admission rate was 3.74 per 100 NHC admissions in the pediatric age group. Infections predominated in younger patients, whereas cardiac complications, diabetes mellitus, and cholecystitis/cholelithiasis were more common in older patients. Hospital admissions for cardiac complications and diabetes mellitus in pediatric patients with thalassemia decreased over the study period. The annual hospital admission cost ranged from 8.19 to 12.01 million US dollars, with one-third attributed to iron chelation. In summary, thalassemia poses a significant healthcare challenge in Thai children, characterized by high admission rates and costs. While infections predominate in younger patients, cardiac complications and diabetes mellitus are more common in older individuals. The diminishing admissions for these complications suggest the successful implementation of iron chelation medications.
Full article
(This article belongs to the Section Conventional Treatment of Thalassemia)
►▼
Show Figures

Figure 1
Open AccessReview
Challenges of Iron Chelation in Thalassemic Children
by
Alkistis Adramerina and Marina Economou
Thalass. Rep. 2024, 14(1), 1-9; https://doi.org/10.3390/thalassrep14010001 - 01 Feb 2024
Abstract
Thalassemia treatment still relies on supportive care, mainly including blood transfusion and iron chelation therapy. Iron chelation is considered the main factor responsible for the marked improvement in survival rates of thalassemic patients. Hemosiderosis may be prevented if appropriate chelation therapy is offered
[...] Read more.
Thalassemia treatment still relies on supportive care, mainly including blood transfusion and iron chelation therapy. Iron chelation is considered the main factor responsible for the marked improvement in survival rates of thalassemic patients. Hemosiderosis may be prevented if appropriate chelation therapy is offered from early childhood, with timely dose adjustments according to changing body weight and close monitoring of organ iron load. With three iron chelators currently available, the choice of appropriate chelation, either as monotherapy or combined therapy, should be individualized depending on the iron overload of target organs, patient’s age, presence of adverse events and compliance issues, given known limitations related to each agent’s administration.
Full article
(This article belongs to the Section Conventional Treatment of Thalassemia)
Open AccessCommunication
β Thalassemia Mutation Flow in Indonesia: A Migration Perspective
by
Lantip Rujito, Ziske Maritska and Abdul Salam Sofro
Thalass. Rep. 2023, 13(4), 253-261; https://doi.org/10.3390/thalassrep13040022 - 15 Dec 2023
Abstract
►▼
Show Figures
Indonesia is a large island country with a wide variety of ethnic groups. As part of the thalassemia country belt, Indonesia has alleles that are as distinctive as those found in other parts of Southeast Asia. The journey of ancestors in the prehistoric
[...] Read more.
Indonesia is a large island country with a wide variety of ethnic groups. As part of the thalassemia country belt, Indonesia has alleles that are as distinctive as those found in other parts of Southeast Asia. The journey of ancestors in the prehistoric period and the massive increase in human exchange in the last decade have formed the current population of Indonesia. The mutants of the beta-thalassemia allele brought by those predecessors can be seen from the traces of their journey. This paperdescribes the flow gene according to the type of mutations of beta-thalassemia in the country.
Full article

Figure 1
Open AccessSystematic Review
Amlodipine Therapy in β-Thalassemia Patients: A Systematic Review and Meta-Analysis on Ferritin Levels and Liver MRI T2*
by
Aily Aliasgharian, Hossein Karami, Mohammad Zahedi, Reza Jahanshahi, Hossein Bakhtiari-Dovvombaygi, Amirreza Nasirzadeh, Mohammad Naderisorki, Mehrnoush Kosaryan, Ebrahim Salehifar, Mobin Ghazaiean, Saeid Bitaraf and Hadi Darvishi-Khezri
Thalass. Rep. 2023, 13(4), 241-252; https://doi.org/10.3390/thalassrep13040021 - 11 Dec 2023
Abstract
►▼
Show Figures
Background and aim: We conducted a review to determine the efficacy of amlodipine alongside iron chelators on serum ferritin levels and liver T2-weighted magnetic resonance imaging (MRI T2*) in β-thalassemia patients. Methods: Systematic search was conducted in multiple databases, including Web of Science,
[...] Read more.
Background and aim: We conducted a review to determine the efficacy of amlodipine alongside iron chelators on serum ferritin levels and liver T2-weighted magnetic resonance imaging (MRI T2*) in β-thalassemia patients. Methods: Systematic search was conducted in multiple databases, including Web of Science, PubMed, Scopus, Embase, Cochrane Library, ClinicalTrials.gov, the Iranian Registry of Clinical Trials (IRCT), ProQuest, OpenGrey, and Web of Science Conference Proceedings Citation Index. The search was closed in January 2023. Primary outcomes were comprised of liver MRI T2* (millisecond (msec)) and serum ferritin levels (ng/mL). Results: Seven studies (n = 227) were included in the study. The pooled Cohen’s d for serum ferritin was estimated at −0.46, 95% confidence interval (CI) −1.11 to 0.19 and p = 0.16 (I2 86.23%, p < 0.0001). The pooled mean difference for serum ferritin was −366.44 ng/mL, 95% CI −844.94 to 112.05, and p = 0.13 (I2 81.63%, p < 0.0001). After a meta-regression based on the length of using amlodipine, a coefficient for the mean difference was also −23.23 ng/mL and 95% CI −155.21 to 108.75. The coefficient obtained from a meta-regression as per the amlodipine dose at 5 mg/day than 2.5 to 5 mg/day anchored at −323.49 ng/mL and 95% CI −826.14 to 1473.12. A meta-regression according to the baseline values of serum ferritin discovered a coefficient of 1.25 ng/mL and 95% CI 0.15 to 2.35. Based on two included studies (n = 96), the overall Cohen’s d for liver MRI T2* was 2.069, 95% CI −0.896 to 5.035, and p = 0.17 (I2 96.31%, p< 0.0001). The synthesized mean difference for liver MRI T2* was 8.76 msec, 95% CI −4.16 to 21.67, and p = 0.18 (I2 98.38%, p < 0.000). Conclusion: At a very low level of evidence, probably using amlodipine at a dose of 2.5 to 5 mg a day, up to a year, alongside iron chelators slightly decreases serum ferritin levels in iron-overloaded thalassemia cases by nearly 366 ng/mL (23 ng/mL per month). The liver MRI T2* might also rise to 8.76 msec upon co-therapy with amlodipine.
Full article
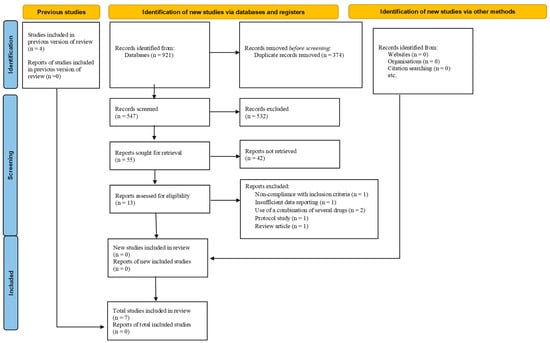
Figure 1
Open AccessArticle
Association of Bone Disorder and Gene Polymorphism of PPAR-γ Pro12 Ala in Egyptian Children with β-Thalassemia
by
Ahmed M. Abdel Hamied, Heba Mostafa Ahmed, Dina H. Eldahshan, Dalia S. Morgan, Abdel Meged A. Abdel Meged, Marwa O. Elgendy, Mohamed S. Imam, Turki A. H. Alotaibi, Majed M. S. Alotaibi, Manal T. N. Alotaibi, Sarah S. S. Alshalan and Sara O. Elgendy
Thalass. Rep. 2023, 13(4), 230-240; https://doi.org/10.3390/thalassrep13040020 - 30 Sep 2023
Abstract
β-thalassemia is a genetic disorder affecting chromosome 16, inherited from one or both parents. In spite of the improved treatment of the hematological disorder and its complications, β-thalassemic patients still exhibit an imbalance in bone mineral turnover, resulting in diminished bone mineral density
[...] Read more.
β-thalassemia is a genetic disorder affecting chromosome 16, inherited from one or both parents. In spite of the improved treatment of the hematological disorder and its complications, β-thalassemic patients still exhibit an imbalance in bone mineral turnover, resulting in diminished bone mineral density (BMD), more evident in the lumbar spine. The purpose of this study was to investigate the association between genetic polymorphism of the PPAR-γ gene and the presence of osteopenia or osteoporosis in children with β-thalassemia. This case–control study was conducted on 50 children with β-thalassemia from the pediatric hematology unit of Beni-Suef University Hospital, including 50 healthy children as the control group. The age range was 8 to 18 years. Samples of patients and control subjects were analyzed for the presence of polymorphisms of the PPAR-γ gene and other blood labs. An assay of BMD measure using dual-energy X-ray absorptiometry (DXA) was performed to investigate osteopenia or osteoporosis. Statistical analysis was used to investigate the relationship between the risk of osteopenia or osteoporosis and the presence of PPAR-γ Pro12Ala gene polymorphism. Eighteen (eleven males and seven females) of fifty patients (representing 36% of the patients group) have osteopenia with low bone mineral density (Z-score is −1 or less than 1). There was no statistically significant difference between BMD measurements in males and females. By comparing the frequency of 12 Ala gene polymorphisms between the patient group and the control group, we found that no statistically significant difference was detected. The BMD values were not significantly different between the groups of PPAR-γ Pro12Ala gene polymorphism. In conclusion, decreased BMD levels are frequent in β-thalassemia patients. PPAR-γ Pro12Ala gene polymorphism is not common in Egyptian patients with β-thalassemia. No significant relationship was found between the PPAR-γ Pro12Ala gene polymorphism and low BMD levels or osteopenia in Egyptian β-thalassemia patients. However, further studies on a larger population of Egyptian patients are needed to confirm this finding.
Full article
Open AccessReview
Infection and Potential Challenge of Childhood Mortality in Sickle Cell Disease: A Comprehensive Review of the Literature from a Global Perspective
by
Tarun Sahu, Babita Pande, Henu Kumar Verma, L V K S Bhaskar, Meenakshi Sinha, Ramanjan Sinha and Pasupuleti Visweswara Rao
Thalass. Rep. 2023, 13(3), 206-229; https://doi.org/10.3390/thalassrep13030019 - 30 Aug 2023
Cited by 2
Abstract
Sickle cell disease (SCD) is a complex genetic disorder associated with multiple clinical manifestations, including increased susceptibility to bacterial and viral infections. This review article presents a comprehensive analysis of the current literature obtained from various online databases focusing on the relationship between
[...] Read more.
Sickle cell disease (SCD) is a complex genetic disorder associated with multiple clinical manifestations, including increased susceptibility to bacterial and viral infections. This review article presents a comprehensive analysis of the current literature obtained from various online databases focusing on the relationship between SCD and infections caused by specific pathogens, such as pneumonia- and influenza-causing pathogens, Escherichia coli, Staphylococcus aureus, parvovirus, and hepatitis viruses. We discuss the underlying mechanisms that contribute to the increased susceptibility of individuals with SCD to these infections, primarily related to the pathophysiology of variant hemoglobin (HbSS) and its impact on vascular occlusion, hemolysis, functional asplenia, and immune deficiency. Moreover, we highlight the significant burden of infections on SCD patients, particularly children under five years of age, where they are the leading cause of morbidity and mortality. Additionally, we address the challenges faced in attempts for reducing the global mortality rate associated with SCD, particularly in low-income countries, where factors such as increased pathogen exposure, co-morbidities like malnutrition, lower vaccination rates, and limited healthcare facilities contribute to the high disease burden. This review emphasizes the need for targeted interventions, improved healthcare access, vaccination programs, and infection prevention strategies to alleviate the impact of infections on individuals with SCD and reduce the global mortality rates associated with the disease.
Full article
(This article belongs to the Section Quality of Life)
►▼
Show Figures
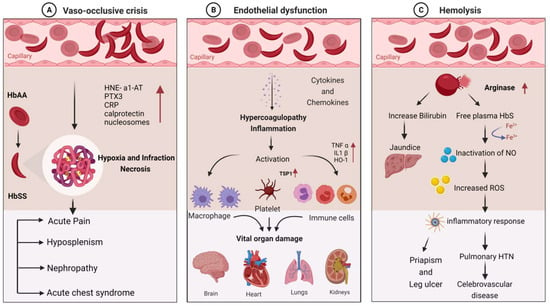
Figure 1
Open AccessReview
Association between Glomerular Filtration Rate and β-Thalassemia Major: A Systematic Review and Meta-Analysis
by
Shahad Saif Khandker, Nurani Jannat, Deepannita Sarkar, Alif Hasan Pranto, Ismoth Ara Hoque, Jemema Zaman, Md. Nizam Uddin and Ehsan Suez
Thalass. Rep. 2023, 13(3), 195-205; https://doi.org/10.3390/thalassrep13030018 - 29 Aug 2023
Abstract
►▼
Show Figures
Thalassemia is one of the most prevalent genetic disorders worldwide and has previously been found to have an association with several physiological and organ complications. Several studies have found both its positive and inverse correlation with the glomerular filtration rate (GFR). Therefore, in
[...] Read more.
Thalassemia is one of the most prevalent genetic disorders worldwide and has previously been found to have an association with several physiological and organ complications. Several studies have found both its positive and inverse correlation with the glomerular filtration rate (GFR). Therefore, in this meta-analysis, we tried to assess the accurate correlation of β-thalassemia major (β-TM) with GFR. We searched in Google Scholar, PubMed, and ScienceDirect, and from the initial 96 articles, we finally included 12 studies. The quality and publication bias assessment confirmed that all the studies were of high to moderate quality with no publication bias. The main outcome of the mean difference (MD) was −6.94, 95%CI: −20.69, 6.80 (p < 0.00001), which indicated a negative correlation of the GFR with β-TM. The sensitivity analyses found one study to be a slight outlier, and reanalyzing the data excluding that study, an MD was achieved of −16.46, 95%CI: −26.81, −6.11 (p < 0.00001), which provides even stronger support for our main outcome. Our result determined that the GFR is generally higher in healthy people as compared to β-TM patients.
Full article

Figure 1
Open AccessReview
Understanding the Intricacies of Iron Overload Associated with β-Thalassemia: A Comprehensive Review
by
Subhangi Basu, Motiur Rahaman, Tuphan Kanti Dolai, Praphulla Chandra Shukla and Nishant Chakravorty
Thalass. Rep. 2023, 13(3), 179-194; https://doi.org/10.3390/thalassrep13030017 - 03 Jul 2023
Abstract
β-thalassemia, a congenital genetic hematological disorder characterized by the decrease or absence of β-globin chains, leads to a decrease in levels of Hemoglobin A. The affected individuals can be categorized into two cohorts based on transfusion dependency: transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia
[...] Read more.
β-thalassemia, a congenital genetic hematological disorder characterized by the decrease or absence of β-globin chains, leads to a decrease in levels of Hemoglobin A. The affected individuals can be categorized into two cohorts based on transfusion dependency: transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT). Remarkably, despite the primary pathology lying in β-globin chain depletion, β-thalassemia also exhibits an intriguing association with iron overload. Iron metabolism, a tightly regulated physiological process, reveals a complex interplay in these patients. Over time, both cohorts of β-thalassemic individuals develop iron overload, albeit through distinct mechanisms. Addressing the diverse complications that arise due to iron overload in β-thalassemic patients, the utilization of iron chelators has gained a lot of significance. With varying efficacies, routes of administration, and modes of action, different iron chelators offer unique benefits to patients. In the Indian context, three commercialized iron chelators have emerged, showcasing a high adherence rate to iron chelator-based treatment regimens among β-thalassemic individuals. In this review, we explore the intriguing connection between β-thalassemia and iron overload, shedding light on the intricate mechanisms at play. We delve into the intricacies of iron metabolism, unveiling the distinct pathways leading to iron accumulation in these patients. Additionally, the therapeutic efficacy of different iron chelators in managing iron overload complications is mentioned briefly, along with the guidelines for their usage in India. Through this comprehensive analysis, we aim to deepen our understanding of β-thalassemia and iron overload, paving the way for optimized treatment strategies. Ultimately, our findings provide valuable insights into improving the care and outcomes of individuals affected by β-thalassemia.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes in Developing Countries: Has Anything Changed?)
►▼
Show Figures
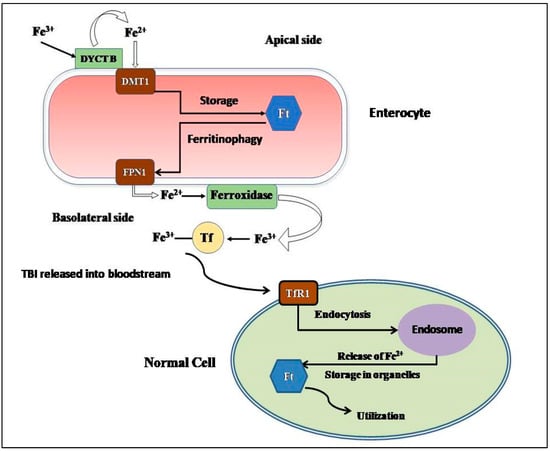
Figure 1
Open AccessArticle
Molecular Epidemiology of HCV Infection among Multi-Transfused β-Thalassemia Patients in Eastern India: A Six-Year Observation
by
Supradip Dutta, Aritra Biswas, Sagnik Bakshi, Promisree Choudhury, Raina Das, Shreyasi Nath, Prosanto Chowdhury, Maitreyee Bhattacharyya, Sharmistha Chakraborty, Shanta Dutta and Provash Chandra Sadhukhan
Thalass. Rep. 2023, 13(3), 165-178; https://doi.org/10.3390/thalassrep13030016 - 25 Jun 2023
Abstract
►▼
Show Figures
Background: HCV infection is very common in multi-transfused β-thalassemia patients who need regular blood transfusions. Aim: The study was conducted to determine the epidemiology of HCV in multi-transfused β-thalassemia patients in West Bengal, India. Methods: Over a span of six years, blood samples
[...] Read more.
Background: HCV infection is very common in multi-transfused β-thalassemia patients who need regular blood transfusions. Aim: The study was conducted to determine the epidemiology of HCV in multi-transfused β-thalassemia patients in West Bengal, India. Methods: Over a span of six years, blood samples were collected from HCV sero-reactive β-thalassemia patients and processed for viral RNA isolation followed by nested RT-PCR for qualitative viremia detection. The HCV genotype was determined by amplifying the partial HCV core gene by nested RT-PCR followed by DNA sequencing and NCBI genotyping tools. Phylogenetic and phylogeographic studies were performed with MEGA-X and BEAST software, respectively. Results: Out of 917 multi-transfused HCV sero-reactive β-thalassemia patients, 598 (65.21%) were HCV RNA positive while 250 (41.80%) had spontaneously cleared the virus. A significant percentage of male patients from rural areas (p = 0.042) and economically backward class (p = 0.002) were at higher risk of HCV infection. Female thalassemia patients and individuals belonging to ages 11–15 years had higher chances of spontaneous clearance. The most prevalent circulatory HCV genotype was 3a (78.26%) followed by 1b (12.04%). Phylogeographic analyses revealed that the 3a strains share genomic similarities with strains from Pakistan, Sri Lanka, and Thailand, whereas the 1b strains share similarities with strains from Thailand, Vietnam, Russia, and China. Uncommon HCV subtypes 3g and 3i were also detected. Conclusion: The high prevalence of HCV infection among β-thalassemia patients of West Bengal, India indicates NAT-based assays should be implemented for HCV screening in donor blood to eliminate HCV by 2030.
Full article

Figure 1
Open AccessReview
The Benign Clone Causing Aplastic Anaemia
by
Shaun R. McCann and Andrea Piccin
Thalass. Rep. 2023, 13(2), 157-164; https://doi.org/10.3390/thalassrep13020015 - 12 Jun 2023
Abstract
Severe Aplastic Anaemia (SAA) is a rare benign disease but carries a high-mortality rate unless treated in a specialised centre. Overwhelming laboratory and clinical evidence points to an autoimmune pathogenesis; although, the aetiology remains obscure in the majority of cases. The differential diagnosis
[...] Read more.
Severe Aplastic Anaemia (SAA) is a rare benign disease but carries a high-mortality rate unless treated in a specialised centre. Overwhelming laboratory and clinical evidence points to an autoimmune pathogenesis; although, the aetiology remains obscure in the majority of cases. The differential diagnosis in older patients is problematical and a diagnosis of hypoplastic myelodysplasia remains difficult. This review points out the difficulty in diagnosis without a specific test. Future research needs to define a specific diagnostic test and refine therapeutic interventions.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes as a Benign Cancer of Hematopoietic Stem Cells)
►▼
Show Figures
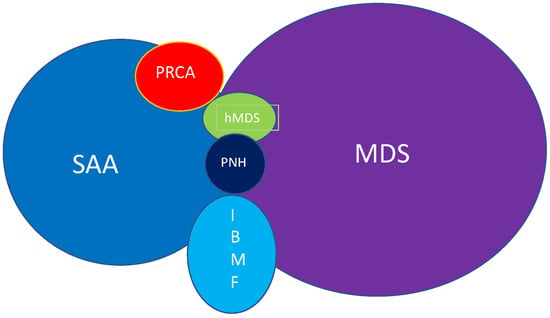
Figure 1
Open AccessCase Report
HbAdrian (α1:c.251del, p.Leu84Argfs*19)—A Novel Pathogenic Variant in the α1-Globin Gene Associated with Microcytosis from the North of Iran
by
Hossein Jalali, Hossein Karami, Mahan Mahdavi and Mohammad Reza Mahdavi
Thalass. Rep. 2023, 13(2), 152-156; https://doi.org/10.3390/thalassrep13020014 - 01 Jun 2023
Abstract
►▼
Show Figures
Background: Alpha thalassemia is one of the most common human genetic abnormalities. More than 400 different variations of the α-globin protein have been introduced, most of which are not associated with noticeable clinical manifestations. The identification of all variants of Hb in different
[...] Read more.
Background: Alpha thalassemia is one of the most common human genetic abnormalities. More than 400 different variations of the α-globin protein have been introduced, most of which are not associated with noticeable clinical manifestations. The identification of all variants of Hb in different regions helps in acquiring comprehensive knowledge concerning thalassemia disease, and it can be used in preventive programs as well as prenatal diagnosis (PND). Aims: In the present study, we describe a new α1 gene mutation that leads to a frameshift after codon 83. Methods: As a plan for a national screening program of thalassemia, routine cell blood count (CBC) and Hb capillary electrophoresis tests were applied. After taking written informed consent, genomic DNA was extracted, and, for identifying common Mediterranean α-Globin gene deletion, multiplex Gap-PCR was performed; for detecting other mutations on α- and β-Globin genes, a DNA sequencing method was used. Results: The results of CBC and capillary electrophoresis tests showed microcytosis in a female subject. The sequencing of the α-Globin gene showed that the case is heterozygote for a single-nucleotide deletion at codon 83 of the α1-Globin Gene. We named this mutation Hb Adrian (α1: c.251–T), which is a novel mutation. The mentioned mutation was also detected in the subject’s mother. Conclusions: The introduced mutation (Hb Adrian) leads to a frameshift change that produces a protein with 100 amino acids, which in comparison to a normal α-chain is shorter, and its amino acids are altered after codon 83. This hemoglobin is undetectable via the use of electrophoresis. Although no major hematological abnormalities were observed in the carriers, Hb Adrian should be considered in screening programs to help prevent Hb H disease in high-risk couples.
Full article
Figure 1
Open AccessPerspective
What Is the Relevance of Murburn Concept in Thalassemia and Respiratory Diseases?
by
Kelath Murali Manoj
Thalass. Rep. 2023, 13(2), 144-151; https://doi.org/10.3390/thalassrep13020013 - 12 May 2023
Abstract
Murburn concept is a novel perspective for understanding cellular function, deeming cells as simple chemical engines (SCE) that are powered by redox reactions initiated by effective charge separation (ECS). The 1-electron active diffusible reactive (oxygen) species, or DR(O)S, equilibriums involved in these processes
[...] Read more.
Murburn concept is a novel perspective for understanding cellular function, deeming cells as simple chemical engines (SCE) that are powered by redox reactions initiated by effective charge separation (ECS). The 1-electron active diffusible reactive (oxygen) species, or DR(O)S, equilibriums involved in these processes are also crucial for homeostasis, coherently networking cells, and rendering electromechanical functions of sensing and responding to stimuli. This perspective presents the true physiological function of oxygen, which is to enable ECS and the generation of DR(O)S. Therefore, DR(O)S must now to be seen as the quintessential elixir of life, although they might have undesired effects (i.e., the traditionally perceived oxidative stress) when present in the wrong amounts, places and times. We also elaborated that tetrameric hemoglobin (Hb) is actually an ATP-synthesizing murzyme (an enzyme working via murburn concept) and postulated that several post-translational modifications (such as glycation) on Hb could result from murburn activity. Murburn perspective has also enabled the establishment of a facile rationale explaining the sustenance of erythrocytes for 3–4 months, despite their lacking nucleus or mitochondria (to coordinate their various functions and mass-produce ATP, respectively). Although thalassemia has its roots in genetic causation, the new awareness of the mechanistic roles of oxygen-hemoglobin-erythrocyte trio significantly impacts our approaches to interpreting research data and devising therapies for this malady. These insights are also relevant in other clinical manifestations that involve respiratory distress (such as asthma, lung cancer, COVID-19 and pneumonia) and mitochondrial diseases. Herein, these contexts and developments are briefly discussed.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes in Developing Countries: Has Anything Changed?)
►▼
Show Figures
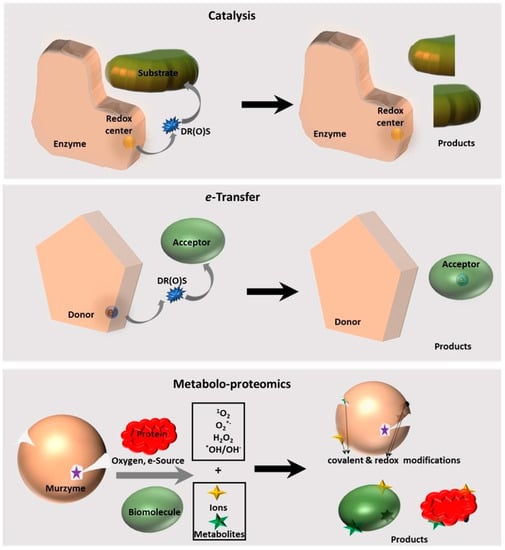
Figure 1
Open AccessArticle
Spectrum of Thalassemia and Hemoglobinopathy Using Capillary Zone Electrophoresis: A Facility-Based Single Centred Study at icddr,b in Bangladesh
by
Anamul Hasan, Jigishu Ahmed, Bikash Chandra Chanda, Maisha Aniqua, Raisa Akther, Palash Kanti Dhar, Kazi Afrin Binta Hasan, Abdur Rouf Siddique, Md. Zahidul Islam, Sharmine Zaman Urmee and Dinesh Mondal
Thalass. Rep. 2023, 13(2), 131-143; https://doi.org/10.3390/thalassrep13020012 - 10 May 2023
Abstract
Background: Although the global thalassemia zone covers Bangladesh, there are very limited studies conducted in this region. Therefore, the focus of our study is to understand the prevalence and burden of thalassemia and hemoglobinopathy in Bangladesh. Methods: The analysis was based on a
[...] Read more.
Background: Although the global thalassemia zone covers Bangladesh, there are very limited studies conducted in this region. Therefore, the focus of our study is to understand the prevalence and burden of thalassemia and hemoglobinopathy in Bangladesh. Methods: The analysis was based on a retrospective evaluation of laboratory diagnoses between 2007 January and 2021 October. A total of 8503 specimens were sampled and analyzed which were either referred by corresponding physicians or self-referred. This was neither any epidemiological nationwide survey nor was the study population chosen randomly. Hematological data were obtained through capillary zone electrophoresis and corresponding complete blood count. Results: 1971 samples (~23.18% of the total) were found with at least one inherited hemoglobin disorder. The most common hemoglobin disorder observed was the hemoglobin E (Hb E) trait (10.67%), followed by the β-thalassemia trait (8.4%), homozygotic Hb E (1.59%), and Hb E/β-thalassemia (1.58%). Other variants found in this study with minimal percentages were Hb N-Seattle, Hb S, Hb D-Punjab, Hb Lepore, Hb C, Hb Hope, Hb H, and hereditary persistence of fetal hemoglobin. Discussion: The pattern of thalassemia and hemoglobinopathy in our study is diverse and heterogeneous. A broad and detailed spectrum of such inherited hemoglobin disorders will ultimately be helpful in implementing nationwide thalassemia management and strategy policy in Bangladesh.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes in Developing Countries: Has Anything Changed?)
►▼
Show Figures
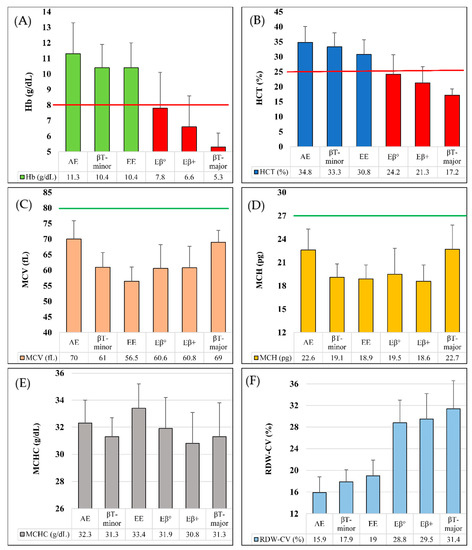
Figure 1
Open AccessFeature PaperReview
Bone Marrow Transplantation in Nonmalignant Haematological Diseases: What Have We Learned about Thalassemia?
by
Luca Castagna, Stefania Tringali, Giuseppe Sapienza, Roberto Bono, Rosario Di Maggio and Aurelio Maggio
Thalass. Rep. 2023, 13(2), 122-130; https://doi.org/10.3390/thalassrep13020011 - 24 Apr 2023
Abstract
Allogeneic stem cell transplantation remains the only therapy for congenital, severe haemoglobinopathies that is able to reverse the pathological phenotype. In the severe form of thalassemia, regular transfusions are needed early in life. This population of patients could benefit from allo-SCT. However, the
[...] Read more.
Allogeneic stem cell transplantation remains the only therapy for congenital, severe haemoglobinopathies that is able to reverse the pathological phenotype. In the severe form of thalassemia, regular transfusions are needed early in life. This population of patients could benefit from allo-SCT. However, the great efficacy of transplantation must be counterbalanced by the mortality and morbidity related to the procedure. In this short review, we reviewed the most recent data in the field of transplantation in transfusion-dependent thalassemia (TDT), highlighting the factors that have a major impact on outcomes.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes as a Benign Cancer of Hematopoietic Stem Cells)
Open AccessArticle
Effect of HFE Gene Mutations on Iron Metabolism of Beta-Thalassemia Carriers
by
María E. Mónaco, Natalia S. Alvarez Asensio, Cecilia Haro, Magdalena M. Terán, Miryam E. Ledesma Achem, Blanca A. Issé and Sandra S. Lazarte
Thalass. Rep. 2023, 13(1), 113-121; https://doi.org/10.3390/thalassrep13010010 - 17 Mar 2023
Cited by 1
Abstract
The human hemochromatosis protein HFE is encoded by the HFE gene and participates in iron regulation. The aim of this study was to detect the most frequent HFE gene mutations in a control population and in β-thalassemia trait (BTT) carriers, and to study
[...] Read more.
The human hemochromatosis protein HFE is encoded by the HFE gene and participates in iron regulation. The aim of this study was to detect the most frequent HFE gene mutations in a control population and in β-thalassemia trait (BTT) carriers, and to study their relationship with iron metabolism. Total blood count, hemoglobin electrophoresis at alkaline pH, HbA2 quantification, iron (Fe), total Fe binding capacity and ferritin were assayed. HFE gene mutations were analyzed by real-time PCR. A total of 119 individuals (69 normal and 50 BTT) were examined. In the control group, 9% (6/69) presented a codon 282 heterozygous mutation (C282Y), and 19% a codon 63 mutation (H63D) (13/69, 11 heterozygotes and 2 homozygotes). In the BTT group, 3 carriers (6%) were heterozygous for C282Y, 14 (28%) for H63D, 1 (2%) for a codon 65 mutation and 1 (2%) was H63D and C282Y double heterozygous. Control group Fe metabolism did not show significant differences (p > 0.05) according to whether or not they carried an HFE gene mutation; while the BTT group with and without HFE mutation showed higher Fe and ferritin than the control group (p < 0.05). However, no increases in iron parameters were detected in BTT carriers that simultaneously exhibited an H63D mutation compared to BTT subjects without a mutation. Therefore, the iron metabolism alterations observed in BTT carriers could not be attributed to the presence of HFE gene mutations. It is likely that BTT individuals have other genetic modifiers that affect their iron balance.
Full article
(This article belongs to the Section Innovative Treatment of Thalassemia)
►▼
Show Figures
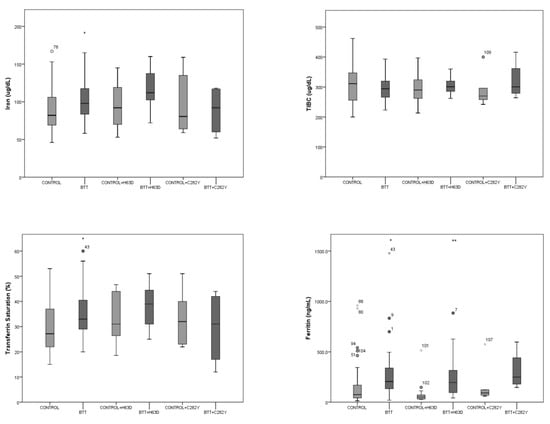
Figure 1
Open AccessArticle
Impact of Genetic Polymorphisms in Modifier Genes in Determining Fetal Hemoglobin Levels in Beta-Thalassemia
by
Poonam Tripathi, Sarita Agarwal, Kausik Mandal, Anshul Gupta and Aditya Narayan Sarangi
Thalass. Rep. 2023, 13(1), 85-112; https://doi.org/10.3390/thalassrep13010009 - 16 Mar 2023
Cited by 1
Abstract
Genetic polymorphisms in Quantitative Trait Loci (QTL) genes such as BCL11A, HBS1L-MYB and KLF1 have been reported to influence fetal hemoglobin (HbF) levels. This prospective study was planned to evaluate the role of genetic polymorphisms in QTL genes as determinant of HbF levels
[...] Read more.
Genetic polymorphisms in Quantitative Trait Loci (QTL) genes such as BCL11A, HBS1L-MYB and KLF1 have been reported to influence fetal hemoglobin (HbF) levels. This prospective study was planned to evaluate the role of genetic polymorphisms in QTL genes as determinant of HbF levels in beta thalassemia major patients. The study was carried out on 100 thalassemia major patients. Blood samples were collected in EDTA and plain vials for biochemical and molecular evaluation. The BCL11A, HBS1L-MYB and KLF1 genotypes were determined using a polymerase chain reaction (PCR)-based method. Red Blood Cell (RBC) indices and HbF levels were assessed. In silico analysis was assessed using loss-of-function tool (Lof Tool). Statistical difference and genetic comparisons between groups were evaluated by using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Comparisons between quantitative variables were carried out after data explored for normality using Kolmogorov–Smirnov test of normality. Logistic regression was used for computation of ORs and 95% CIs (Confidence Interval). We observed association of HbF levels in thalassemia major patients with the polymorphisms in BCL11A (rs11886868 rs7557939; rs1427407 and rs766432) and HBS1L-MYB (rs9399137) gene. The results of this study indicated that the presence of polymorphisms on modifier genes are strongly associated with an increase in HbF levels in thalassemia major patients. Further research with a larger sample size and with other genes of modifier genes is required.
Full article
(This article belongs to the Section Innovative Treatment of Thalassemia)
►▼
Show Figures
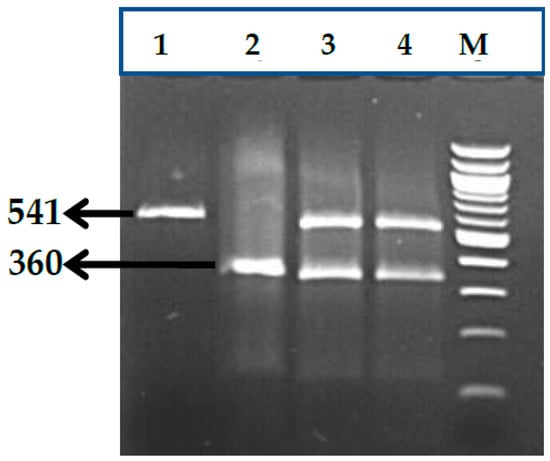
Figure 1
Open AccessPerspective
Highlights on the Luspatercept Treatment in Thalassemia
by
Yesim Aydinok
Thalass. Rep. 2023, 13(1), 77-84; https://doi.org/10.3390/thalassrep13010008 - 20 Feb 2023
Cited by 1
Abstract
Luspatercept has been shown to act as a ligand trap, selectively suppressing the deleterious effects of GDF11 that blocks terminal erythroid maturation, restoring normal erythroid differentiation and improving anemia in animal models of β-thalassemia. Effective doses of luspatercept achieved hemoglobin increase within 7
[...] Read more.
Luspatercept has been shown to act as a ligand trap, selectively suppressing the deleterious effects of GDF11 that blocks terminal erythroid maturation, restoring normal erythroid differentiation and improving anemia in animal models of β-thalassemia. Effective doses of luspatercept achieved hemoglobin increase within 7 days of the first dose, and plasma half-life supports subcutaneously administration every 21 days in adults with β-thalassemia. A Phase 3, placebo-controlled 1-year study with starting dose of 1.0 up to 1.25 mg/kg every 21 days achieved ≥33% reduction in red cell transfusion volume in 21.4% of adult transfusion-dependent β-, HbE/β-thalassemia patients on luspatercept vs. 4.5% on placebo over a fixed 12-week period, and 41.1% of patients in luspatercept vs. 2.7% placebo in any 24-week period. Luspatercept allowed ≥1.0 and ≥1.5 g/dL increase in hemoglobin from baseline in 77% and 52.1% of adult non-transfusion-dependent β-, HbE/β-thalassemia patients vs. 0% placebo over a 12-week interval. Although not significant, a greater improvement in patient-reported outcomes was observed with luspatercept. Luspatercept had a manageable safety profile with notable adverse effects of venous thromboembolism in 3.6% of transfusion-dependent β-thalassemia vs. 0.9% of placebo and extramedullary hematopoiesis in 6% of non-transfusion-dependent β-thalassemia vs. 2% of placebo. The pediatric study started patients’ enrollment.
Full article
(This article belongs to the Special Issue Thalassemia Syndromes as a Benign Cancer of Hematopoietic Stem Cells)
►▼
Show Figures
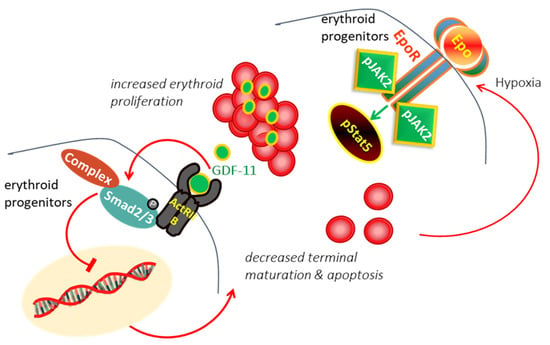
Figure 1
Open AccessArticle
New-Generation Ektacytometry Study of Red Blood Cells in Different Hemoglobinopathies and Thalassemia
by
Elena Krishnevskaya, Marta Molero, Águeda Ancochea, Ines Hernández and Joan-Lluis Vives-Corrons
Thalass. Rep. 2023, 13(1), 70-76; https://doi.org/10.3390/thalassrep13010007 - 16 Feb 2023
Cited by 1
Abstract
Next-generation ektacytometry provided by the osmoscan module of the Laser Optical Rotational Red Cell Analyser (LoRRca) MaxSis is, so far, one of the best complementary diagnostic tools for congenital rare anaemias due to red blood cell defects. Osmotic gradient ektacytometry (OGE) is currently
[...] Read more.
Next-generation ektacytometry provided by the osmoscan module of the Laser Optical Rotational Red Cell Analyser (LoRRca) MaxSis is, so far, one of the best complementary diagnostic tools for congenital rare anaemias due to red blood cell defects. Osmotic gradient ektacytometry (OGE) is currently considered the gold standard for the diagnosis of red cell membrane disorders, especially hereditary spherocytosis (HS). Impairment of red cell deformability, leading to a decrease in red cell survival rate, is the common trait of hereditary haemolytic anaemias; in general, it is the consequence of an abnormal cell shape, increased rigidity or dehydration. Up to now, the next-generation ektacytometry has been mainly used for the differential diagnosis of red blood cell membranopathies, but experience with structural hemoglobinopathies and thalassemia is still scarce. However, recently, many new forms of therapy are being developed for the treatment of hemoglobinopathies, particularly sickle-cell disease and β-thalassemia; clinical interest in ektacytometry is increasing and should be further explored. Here, we have evaluated the OGE profiles provided by the osmoscan module of the LoRRca ektacytometer in 96 patients with different hemoglobinopathies, both structural and thalassemia, with the aim of analysing their usefulness for the early diagnosis of these disorders either individually or in co-inheritance with other hereditary RBC defects. In addition, this study aims to improve our knowledge of the contribution of red cell deformability, osmotic fragility and intracellular viscosity to the physiopathology of haemolysis, especially when these disorders are a cause of rare anaemia. From this study, we conclude that the osmoscan profile provides complementary information on red cell deformability and hydration homeostasis that may contribute to the better understanding of the physiopathology of decreased red cell survival and hemolysis which is present in some patients.
Full article
(This article belongs to the Special Issue Thalassaemia: A Complex Mix of Genetic Entities Challenging Healthcare Providers Globally)
►▼
Show Figures
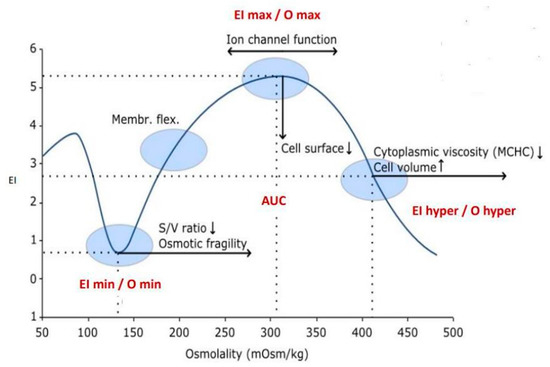
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Topical Collections
Topical Collection in
Thalassemia Reports
Feature Papers in Thalassemia Reports
Collection Editors: Aurelio Maggio, Khaled Musallam