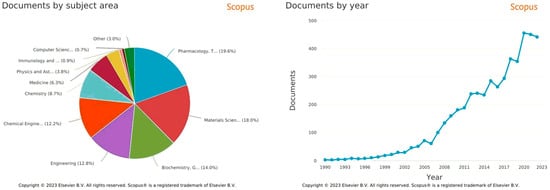
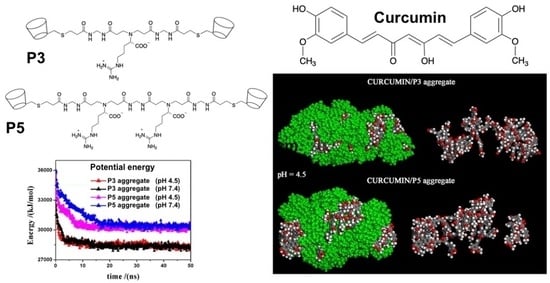
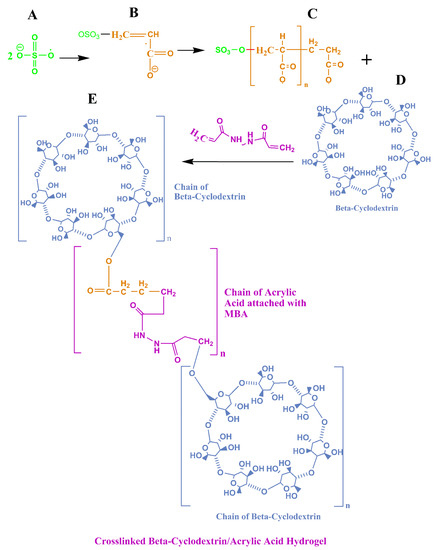
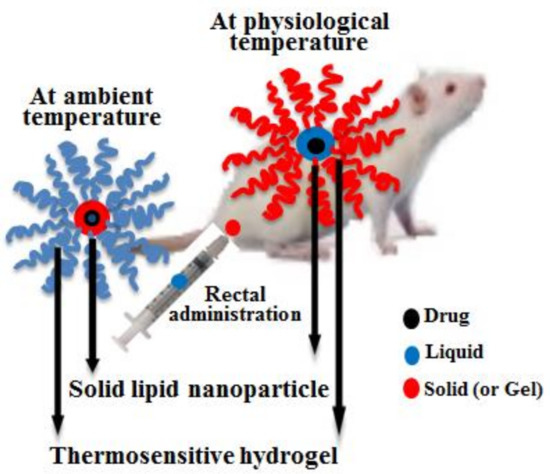
Polymers for Controlled Drug Release
A topical collection in Polymers (ISSN 2073-4360). This collection belongs to the section "Polymer Applications".
Viewed by 19452Editors
Interests: biodegradable polymers; transport phenomena; electrospun fibers; controlled release; polymer blends; composites; water in macromolecular systems; sorption; gas permeability
Special Issues, Collections and Topics in MDPI journals
2. Basic Department of Biotechnology, School of Fundamental Biology and Biotechnology, Siberian Federal University, 79 Svobodnyi Av., 660041 Krasnoyarsk, Russia
Interests: nanomaterials; polymer blends; fiber-filled polymer composites; polymer nanocomposites; aging and degradation; pervaporation phenomena; sorption and diffusion; interpenetrating polymer systems; recyclability and reuse of waste plastics and rubbers; elastomer crosslinking; dual porous nanocomposite scaffolds for tissue engineering; polymer nanocomposites for electronic applications; water purification; energy storage
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Academic and industrial engagement has currently been directed to a required growth in the human lifespan and life quality that is supported by advances in biomedicine, environmental protection, packaging upgrading, etc. The holistic approach to the healthcare therapeutic strategy, innovative packaging development, and eco-friendly material design is based on the creation of conditions for bioactive component release/delivery specifically for drugs in medicine, food-modifying agents in packaging, and pheromones and insecticides in agriculture, correspondingly. Because of the wide implementation of controlled release as the specific phenomenon, it is critical to clearly establish its mechanism and kinetic profile as well as market economic consequences of replacement of traditional medical and packaging formulations by modern polymeric systems, ensuring the prolonged and programmed delivery of bioactive agents.
A series of significant contemplations are outlined in this Collection to establish spatial–temporary patterns for bioactive entities loaded in polymer vehicles of synthetic and bio-based origin. In a release systems design, one of the most important unit operations is the size reduction to the nanoscale and molecular levels. In biomedicine, the final object of drug-controlled delivery application comprises a spatial–temporary pattern setting to carry out the most favorable treatment of patients. Novel miniature therapeutic systems with drug targeting characteristics have irrefutable advantages such as enhanced bioavailability, reduced toxicity, tailored release profiles, improved stability, and others, which take precedence over regular dosage forms. The variety of geometrical forms for polymer therapeutic systems at the submicron levels, namely, spheres, Janus particles, ellipsoid-like beds, rods, ribbon and cylindrical fibers, slabs, and the like, creates a great opportunity to set up original kinetic release profiles combined with improved bio-accessibility for cells and tissues. The next step after nanotechnology engineering includes the principles of polymer science, which coherently seek a relationship between structure–morphology polymer features and drug diffusion transport characteristics. Modeling and experimental studies of transport phenomena should assist the development of next-generation drug release therapeutic systems.
In this collection, it is our aim to provide Polymers MDPI readers with the latest developments in the design, characterization, and applications of controlled release systems with different composition and geometry.
Topics include but are not limited to the following list:
- The fundamental principles and breakthrough innovations of controlled release/delivery as the specific phenomenon occurring in stable (petrol-based) and biodegradable (bio-based) polymers;
- Micro engineering and nanotechnology of polymeric systems procuring the controlled release;
- Elaboration of controlled release micro- and nano-arrays with diverse geometry such as embedded dots, conjugated dendrimers, loaded particles, fibers, slabs;
- Structure–morphology peculiarities of homogeneous, heterogeneous, and multilayered polymer composites as the factor of release profile regulation;
- The impact of the balance of hydrophilicity–hydrophobicity on the mechanism of controlled release for drugs and polymer matrices, and the problem of poor-dissolved drugs with low-dosage activity;
- Specific performance of drug-controlled release systems for gels, elastic, and glassy-state polymer constructs for vehicles performing in different areas of medicine, namely, in ophthalmology, reconstructive surgery, cardiovascular therapy; social and orphan decease treatment, dentistry, neurology, and others;
- Controlled release for tissue engineering, growth factor delivery from ultrathin fibers;
- Controlled release for food packaging as the fundament of active packaging development;
- Controlled release for environmentally benign areas;
- Modeling and computer data operations to create novel polymer release systems with bioactive agent temporal–spatial patterning in the human body, active barrier polymers, and the environment.
Prof. Dr. Alexey Iordanskii
Prof. Dr. Sabu Thomas
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Polymers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.