Plant Virology in Australia
Share This Topical Collection
Editors
 Dr. Cherie Gambley
Dr. Cherie Gambley
 Dr. Cherie Gambley
Dr. Cherie Gambley
E-Mail
Website
Collection Editor
Department of Agriculture and Fisheries, Microbiology and Entomology, Maroochy Research Station, Nambour, QLD 4560, Australia
Interests: plant virology; bacteriology; epidemiology
 Dr. Paul Campbell
Dr. Paul Campbell
 Dr. Paul Campbell
Dr. Paul Campbell
E-Mail
Website
Collection Editor
Department of Agriculture and Fisheries, Microbiology and Entomology, Ecosciences Precinct, Brisbane 4001, QLD, Australia
Interests: plant virology; biotechnology; genetics; botany
Topical Collection Information
Dear Colleagues,
Plant virology research in Australia is diverse, covering aspects from the characterization of new viruses, to detection and diagnoses, epidemiology, and disease management. The varied climate zones, from tropical to temperate, provide opportunities for research in many and varied cropping systems. Recent incursions of previously exotic viruses have also expanded the range of plant virology done in Australia. Improved biosecurity measures through increased diagnostic testing to prevent further exotic incursions have also driven advances in diagnostic development and surveillance.
New technologies using genome library assemblies have advanced virus characterization and led to improved resolution of the virus species present in Australia, including descriptions of previously unknown viruses. Broader adoption of the LAMP technology for virus detection has improved diagnostic capacity within Australia. Approaches to disease management have shifted from traditional on-farm efforts to an improved understanding of the need for an area-wide approach. This includes greater emphasis on the interactions between insect vectors, the environment and the crop and how weather can influence these interactions.
The forthcoming Topical Collection aims to provide a comprehensive summary of recent advances in plant virology in Australia with a focus on newly detected viruses, epidemiology, disease management and diagnostic development. There is particular interest in research on how climate influences virus diseases and novel approaches to virus disease management.
Dr. Cherie Gambley
Dr. Paul Campbell
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Plants is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- epidemiology
- genome characterization
- vector transmission
- LAMP
- HTS
- virus management
- begomovirus
- tospovirus
- tobamovirus
- potyvirus
- carlavirus
Published Papers (6 papers)
Open AccessArticle
Genomic Characterisation of an Isolate of Brassica Yellows Virus Associated with Brassica Weed in Tasmania
by
Muhammad Umar, Tahir Farooq, Robert S. Tegg, Tamilarasan Thangavel and Calum R. Wilson
Cited by 4 | Viewed by 1846
Abstract
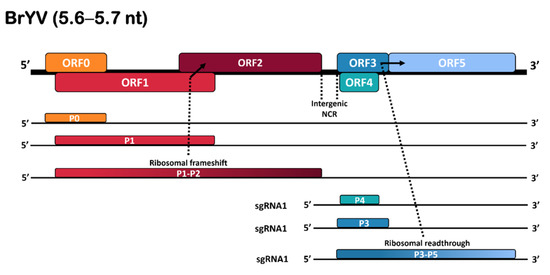
Brassica yellows virus (BrYV), a tentative species in the genus
Polerovirus, of the
Solemoviridae family, is a phloem-restricted and aphid-transmitted virus with at least three genotypes (A, B, and C). It has been found across mainland China, South Korea, and Japan. BrYV
[...] Read more.
Brassica yellows virus (BrYV), a tentative species in the genus
Polerovirus, of the
Solemoviridae family, is a phloem-restricted and aphid-transmitted virus with at least three genotypes (A, B, and C). It has been found across mainland China, South Korea, and Japan. BrYV was previously undescribed in Tasmania, and its genetic variability in the state remains unknown. Here, we describe a near-complete genome sequence of BrYV (genotype A) isolated from
Raphanus raphanistrum in Tasmania using next-generation sequencing and sanger sequencing of RT-PCR products. BrYV-Tas (GenBank Accession no. OM469309) possesses a genome of 5516 nucleotides (nt) and shares higher sequence identity (about 90%) with other BrYV isolates. Phylogenetic analyses showed variability in the clustering patterns of the individual genes of BrYV-Tas. Recombination analysis revealed beginning and ending breakpoints at nucleotide positions 1922 to 5234 nt, with the BrYV isolate LC428359 and BrYV isolate KY310572 identified as major and minor parents, respectively. Results of the evolutionary analysis showed that the majority of the codons for each gene are evolving under purifying selection, though a few codons were also detected to have positive selection pressure. Taken together, our findings will facilitate an understanding of the evolutionary dynamics and genetic diversity of BrYV.
Full article
►▼
Show Figures
Open AccessArticle
Investigating the Longevity and Infectivity of Cucumber green mottle mosaic virus in Soils of the Northern Territory, Australia
by
David Lovelock, Sharl Mintoff, Nadine Kurz, Merran Neilsen, Shreya Patel, Fiona Constable and Lucy Tran-Nguyen
Cited by 6 | Viewed by 2324
Abstract
Cucumber green mottle mosaic virus (CGMMV) is a
Tobamovirus of economic importance affecting cucurbit crops and Asian cucurbit vegetables. CGMMV was detected in the Northern Territory (NT) in September 2014, the first record for Australia, with 26 properties confirmed as of May 2016.
[...] Read more.
Cucumber green mottle mosaic virus (CGMMV) is a
Tobamovirus of economic importance affecting cucurbit crops and Asian cucurbit vegetables. CGMMV was detected in the Northern Territory (NT) in September 2014, the first record for Australia, with 26 properties confirmed as of May 2016. Research was undertaken to determine virus longevity in soils in the NT and investigate the use of disinfectants to remove viable CGMMV from the soil. An in-field trial at 12 months post-quarantine at four properties, and bioassays from collected soils indicate that CGMMV remained viable in at least two of the properties 12 months after plant hosts were removed from the ground. The infectivity of CGMMV from soil was also investigated in two trials with 140 watermelon seeds and 70 watermelon plants sown into CGMMV infested soils with or without the application of the disinfectants Virkon
TM (2%) and Bleach (1%). Watermelons grown in soil, not treated with the Virkon
TM or Bleach, showed CGMMV infection rates of 4% and 2.5% respectively. When Virkon
TM or Bleach was applied, no positive CGMMV detections were observed in the watermelons. This research highlights the importance of proper management of infested properties and the need for on-farm biosecurity to manage CGMMV.
Full article
►▼
Show Figures
Open AccessArticle
The Establishment and Spread of a Newly Introduced Begomovirus in a Dry Tropical Environment Using Tomato Yellow Leaf Curl Virus as a Case Study
by
Cherie Gambley, Peter Nimmo, Janet McDonald and Paul Campbell
Cited by 2 | Viewed by 1761
Abstract
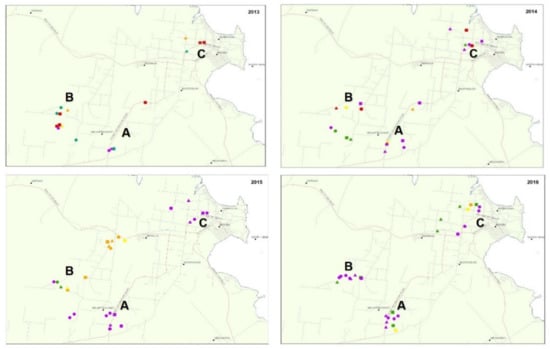
Early detection of tomato yellow leaf curl virus (TYLCV) in a previously unaffected tomato production district in Australia allowed its spread to be evaluated spatially and temporally. The population dynamics of the TYLCV vector,
Bemisia argentifolii (silverleaf whitefly, SLW), were also evaluated. The
[...] Read more.
Early detection of tomato yellow leaf curl virus (TYLCV) in a previously unaffected tomato production district in Australia allowed its spread to be evaluated spatially and temporally. The population dynamics of the TYLCV vector,
Bemisia argentifolii (silverleaf whitefly, SLW), were also evaluated. The district is a dry tropical environment with a clear break to commercial production during the summer wet season. The incidence of TYLCV within crops and its prevalence through the district was influenced by weather, location, vector movements, and the use of Ty-1 virus-resistant hybrids. Rainfall had an important influence, with late summer and early autumn rain suppressing the levels of SLW and, by contrast, a dry summer supporting faster population growth. The use of Ty-1 hybrids appears to have reduced the incidence of TYLCV in this district. There was limited use of Ty-1 hybrids during 2013, and by season end, crops had moderate levels of SLW and high virus incidence. The 2015 and early 2016 season had high SLW populations, but TYLCV incidence was lower than in 2013, possibly due to the widespread adoption of the Ty-1 hybrids reducing virus spread. This study provides valuable epidemiology data for future incursions of begomoviruses, and other viruses spread by SLW.
Full article
►▼
Show Figures
Open AccessReview
Plant Virus and Virus-like Disease Threats to Australia’s North Targeted by the Northern Australia Quarantine Strategy
by
Richard I. Davis, Lynne M. Jones, Bradley Pease, Sandy L. Perkins, Harshitsinh R. Vala, Pere Kokoa, Marilyn Apa and Christopher J. Dale
Cited by 6 | Viewed by 2139
Abstract
The Northern Australia Quarantine Strategy (NAQS) is a biosecurity initiative operated by the Australian federal government’s Department of Agriculture, Water and the Environment (DAWE). It is unique worldwide because it deals specifically with the potential arrival via unregulated pathways of exotic threats from
[...] Read more.
The Northern Australia Quarantine Strategy (NAQS) is a biosecurity initiative operated by the Australian federal government’s Department of Agriculture, Water and the Environment (DAWE). It is unique worldwide because it deals specifically with the potential arrival via unregulated pathways of exotic threats from overseas in a vast and sparsely populated region. It aims to protect the nation’s animal- and plant-based production industries, as well as the environment, from incursions of organisms from countries that lie immediately to the north. These are diseases, pests, and weeds present in these countries that are currently either absent from, or under active containment in, Australia and may arrive by natural or human-assisted means. This review article focuses on the plant viruses and virus-like diseases that are most highly targeted by the NAQS program. It presents eight pathogen species/group entries in the NAQS A list of target pathogens, providing an overview of the historical and current situation, and collates some new data obtained from surveillance activities conducted in northern Australia and collaborative work overseas.
Full article
Open AccessReview
Evolution of Plant Virus Diagnostics Used in Australian Post Entry Quarantine
by
Mark Whattam, Adrian Dinsdale and Candace E. Elliott
Cited by 8 | Viewed by 2918
Abstract
As part of a special edition for MDPI on plant virology in Australia, this review provides a brief high-level overview on the evolution of diagnostic techniques used in Australian government Post-Entry Quarantine (PEQ) facilities for testing imported plants for viruses. A comprehensive range
[...] Read more.
As part of a special edition for MDPI on plant virology in Australia, this review provides a brief high-level overview on the evolution of diagnostic techniques used in Australian government Post-Entry Quarantine (PEQ) facilities for testing imported plants for viruses. A comprehensive range of traditional and modern diagnostic approaches have historically been employed in PEQ facilities using bioassays, serological, and molecular techniques. Whilst these techniques have been effective, they are time consuming, resource intensive and expensive. The review highlights the importance of ensuring the best available science and diagnostic developments are constantly tested, evaluated, and implemented by regulators to ensure primary producers have rapid and safe access to new genetics to remain productive, sustainable and competitive.
Full article
Open AccessArticle
Detection and Distribution of Viruses Infecting Garlic Crops in Australia
by
Julia Cremer, Paul Campbell, Visnja Steele, Denis Persley, John Thomas, Stephen Harper and Cherie Gambley
Cited by 9 | Viewed by 4173
Abstract
The distribution of viruses in eastern Australian field garlic was evaluated. Detection assays were developed that involved generic RT-PCR for viruses in the
Allexivirus,
Carlavirus and
Potyvirus genera followed by virus-specific colorimetric dot-blot hybridization. Assays targeted the potyviruses (onion yellow dwarf virus
[...] Read more.
The distribution of viruses in eastern Australian field garlic was evaluated. Detection assays were developed that involved generic RT-PCR for viruses in the
Allexivirus,
Carlavirus and
Potyvirus genera followed by virus-specific colorimetric dot-blot hybridization. Assays targeted the potyviruses (onion yellow dwarf virus (OYDV), shallot yellow stripe virus (SYSV), and leek yellow stripe virus (LYSV)), the carlaviruses (garlic common latent virus (GCLV) and shallot latent virus (SLV)), and the allexiviruses (garlic viruses A, B, C, X (GarVA, -B, -C, -X) and shallot virus X (ShVX)). Virus incidence in crops was consistently high, with most plants infected with at least one virus from each genus. OYDV, LYSV, SLV, and GCLV were commonly detected. Three of the four allexiviruses were in all districts surveyed but varied in incidence, whereas ShVX and SYSV were not detected. There was no association between virus species complement and bulb size, indicating size is not a good predictor of the virus status of planting material. The variation of virus incidence across different Australian growing districts and in different cultivars implies multiple introductions of viruses rather than spread within the country. The genetic diversity observed within coat protein sequences of some virus species also supports multiple separate introductions.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Cowpea Mild Mottle Virus – a Sometimes Problem for French Bean
Authors: Gambley et al.
Affiliation: Department of Agriculture and Fisheries, Microbiology and Entomology, Maroochy Research Station, Nambour 4560, Queensland, Australia
Title: The Establishment and Spread of a Newly Introduced Begomovirus in a Dry Tropics Environment Using Tomato Yellow Leaf Curl Virus as a Case Study
Authors: Gambley et al.
Affiliation: Department of Agriculture and Fisheries, Microbiology and Entomology, Maroochy Research Station, Nambour 4560, Queensland, Australia
Title: The Detection and Distribution of Viruses in Australian Commercial Garlic Crops
Authors: Cremer et al.
Affiliation: University of Queensland