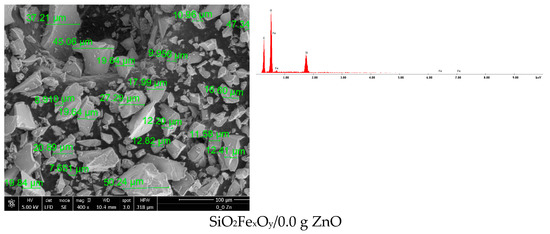
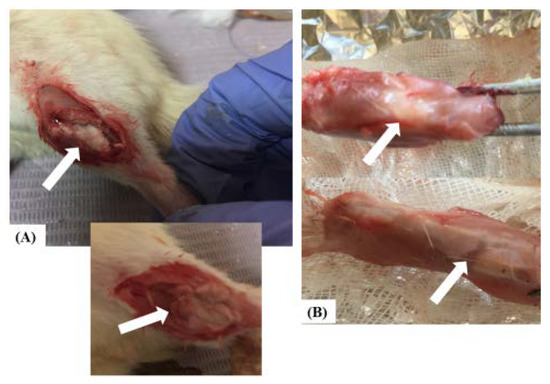
Feature Papers in Nanomedicine and Nanotechnology
A topical collection in Pharmaceutics (ISSN 1999-4923). This collection belongs to the section "Nanomedicine and Nanotechnology".
Viewed by 28249Editors
Interests: nanomedicine; drug delivery; gene therapy; pharmaceutics; pharmaceutical education; topical delivery
Special Issues, Collections and Topics in MDPI journals
Interests: drug delivery; infectious diseases; nanomedicine; nanotechnology; pharmaceutics; women’s health
Special Issues, Collections and Topics in MDPI journals
Interests: topical and transdermal drug delivery; penetration enhancement; nanotechnology; skin permeation mechanisms
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
We are pleased to announce a Special Issue titled “Feature Papers in Nanomedicine and Nanotechnology”. The Special Issue is a collection of papers highlighting and collecting recent progress in nanotechnology and their applications in pharmaceutical, clinical, diagnostic, environmental, and cosmetic fields.
The covered topics include the following:
- Advances in methodologies for characterization of nanocarriers;
- Nanodevices and nanosensors;
- Stability studies;
- Development of new drug delivery systems;
- Nanotoxicity;
- Skin and mucosal application;
- Nanotechnology in tissue engineering and regenerative nanomedicine;
- Functionalization for a selective target;
- Nanodelivery systems in cosmeceutical and nutraceutical field;
- Nanodelivery as a strategy to improve bioavailability;
- Strategies and formulations for controlled drug release;
- In vitro and ex vivo testing;
- In vivo testing;
- Legislation regarding nanocarriers and nanomedicines.
We invite researchers to submit original research articles and reviews in this field. Authors will enjoy high-quality reviewing processes, rapid publication, and high visibility ranging from access to a broad readership.
Prof. Dr. Donatella Paolino
Dr. José das Neves
Prof. Dr. Heather Benson
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pharmaceutics is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- nanomedicine
- nanocarriers
- nanotechnology
- drug delivery
- bioavailability
- formulations
- lipid nanoparticles
- topical delivery
- transdermal drug delivery
- nanotheranostics
- gene therapy