Journal Description
Organoids
Organoids
is an international, peer-reviewed, open access journal on all aspects of organoids published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: first decisions in 16 days; acceptance to publication in 5.8 days (median values for MDPI journals in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Organoids is a companion journal of Cells.
Latest Articles
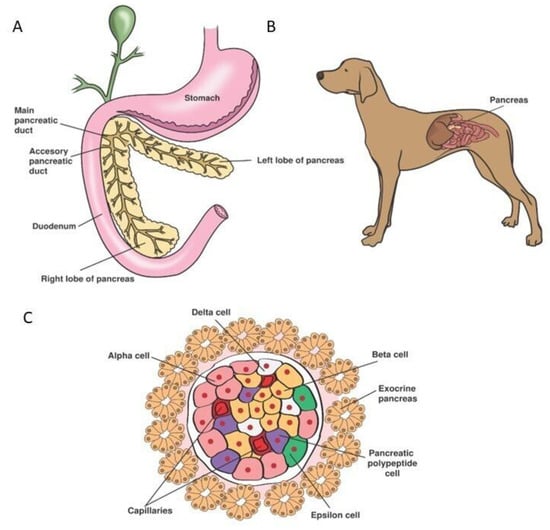
Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy
Organoids 2024, 3(2), 67-82; https://doi.org/10.3390/organoids3020006 - 04 Apr 2024
Abstract
►
Show Figures
For over 150 years, researchers have studied the (patho)physiology of the endocrine pancreas and devised treatment options for diabetes mellitus (DM). However, no cure has been developed so far. In dogs, diabetes mellitus type 1 (T1DM) is the most common presentation. Treatment consists
[...] Read more.
For over 150 years, researchers have studied the (patho)physiology of the endocrine pancreas and devised treatment options for diabetes mellitus (DM). However, no cure has been developed so far. In dogs, diabetes mellitus type 1 (T1DM) is the most common presentation. Treatment consists of twice daily insulin injections, monitored by spatial blood glucose measurements. Even though dogs were instrumental in the discovery of insulin and islet transplantations, the treatment in diabetic dogs has remained unchanged for decades. Providing twice daily insulin injections is demanding for both owners and dogs and may result in hypoglycaemic events, creating the need for new treatment strategies. Novel regenerative medicine-based tools, such as improved β-cell culture protocols and artificial devices, have sparked hope for a cure. In human medicine, emerging technologies such as the transplantation of insulin-producing β-cells, generated by stem cell differentiation, with or without an encapsulation device, are currently tested in phase I/II clinical trials. As the pathogenesis of T1DM is remarkably similar between humans and dogs, novel treatment methods could be implemented in canine medicine. This review briefly summarises the physiology of the canine endocrine pancreas and the pathophysiology of canine DM before exploring current and possible future treatment options for canine DM.
Full article
Open AccessProtocol
Generation of Trophoblast Organoids from Chorionic Villus Sampling
by
Bas van Rijn, Diane Van Opstal, Nicole van Koetsveld, Maarten Knapen, Joost Gribnau and Olivier Schäffers
Organoids 2024, 3(1), 54-66; https://doi.org/10.3390/organoids3010005 - 05 Mar 2024
Abstract
►▼
Show Figures
Studying human placental development and function presents significant challenges due to the inherent difficulties in obtaining and maintaining placental tissue throughout the course of an ongoing pregnancy. Here, we provide a detailed protocol for generating trophoblast organoids from chorionic villi obtained during ongoing
[...] Read more.
Studying human placental development and function presents significant challenges due to the inherent difficulties in obtaining and maintaining placental tissue throughout the course of an ongoing pregnancy. Here, we provide a detailed protocol for generating trophoblast organoids from chorionic villi obtained during ongoing pregnancy. Our method results in efficient generation of trophoblast organoids from chorionic villus sampling, does not require preselection of chorionic villi, and controls contamination of decidual gland organoids. The resulting trophoblast organoids spontaneously form syncytiotrophoblasts that start secreting hCG hormone amongst other placenta-specific factors. Our approach facilitates the generation of trophoblast organoids from a variety of genetic backgrounds, including trisomies and gene mutations, and can be aligned with prenatal diagnostic routines. The protocol requires up to 14 days and can be carried out by users with expertise in cell culture.
Full article
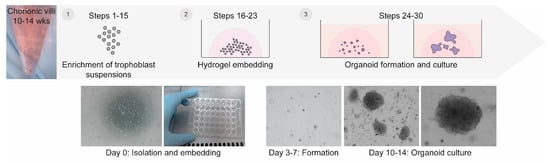
Figure 1
Open AccessArticle
Analysis of Osteosarcoma Cell Lines and Patient Tissue Using a 3D In Vivo Tumor Model—Possible Effects of Punicalagin
by
Anna Rebecca Dorn, Sara Neff, Sophia Hupp, Melissa Engelhardt, Eric Pion, Ulrich Lenze, Carolin Knebel, Anna Duprée, Simone Schewe, Markus Weber, Christian Wulbrand, Axel Hillmann, Florian Weber, Phillip Clarke, Philipp Kainz, Thiha Aung and Silke Haerteis
Organoids 2024, 3(1), 35-53; https://doi.org/10.3390/organoids3010004 - 04 Mar 2024
Abstract
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell
[...] Read more.
Osteosarcomas are the most common primary malignant bone tumors and mostly affect children, adolescents, and young adults. Despite current treatment options such as surgery and polychemotherapy, the survival of patients with metastatic disease remains poor. In recent studies, punicalagin has reduced the cell viability, angiogenesis, and invasion in cell culture trials. The aim of this study was to examine the effects of punicalagin on osteosarcomas in a 3D in vivo tumor model. Human osteosarcoma biopsies and SaOs-2 and MG-63 cells, were grown in a 3D in vivo chorioallantoic membrane (CAM) model. After a cultivation period of up to 72 h, the tumors received daily treatment with punicalagin for 4 days. Weight measurements of the CAM tumors were performed, and laser speckle contrast imaging (LSCI) and a deep learning-based image analysis software (CAM Assay Application v.3.1.0) were used to measure angiogenesis. HE, Ki-67, and Caspase-3 staining was performed after explantation. The osteosarcoma cell lines SaOs-2 and MG-63 and osteosarcoma patient tissue displayed satisfactory growth patterns on the CAM. Treatment with punicalagin decreased tumor weight, proliferation, and tumor-induced angiogenesis, and the tumor tissue showed pro-apoptotic characteristics. These results provide a robust foundation for the implementation of further studies and show that punicalagin offers a promising supplementary treatment option for osteosarcoma patients. The 3D in vivo tumor model represents a beneficial model for the testing of anti-cancer therapies.
Full article
(This article belongs to the Special Issue Advanced Organoids: New Avenues for Understanding Human Anatomy, Physiology and Development)
►▼
Show Figures
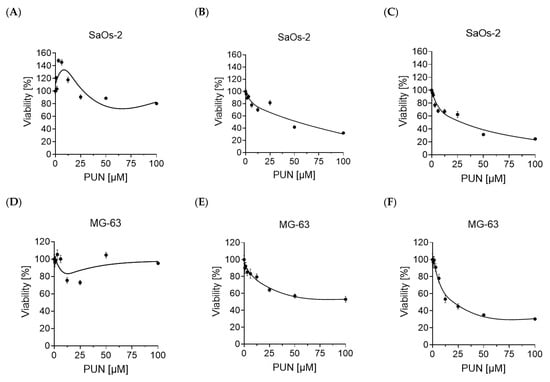
Figure 1
Open AccessEditorial
The Next Generation of Organoids Will Be More Complex and Even Closer to Resembling Real Organs: An Interview with Prof. Dr. Hans Clevers
by
Süleyman Ergün and Organoids Editorial Office
Organoids 2024, 3(1), 32-34; https://doi.org/10.3390/organoids3010003 - 20 Feb 2024
Abstract
In this issue, we are pleased and honored to have an interview with Professor Hans Clevers, who is the Advisory Board Member of Organoids [...]
Full article
Open AccessArticle
Human Nasal Epithelium Organoids for Assessing Neutralizing Antibodies to a Protective SARS-CoV-2 Virus-like Particle Vaccine
by
Julio Carrera Montoya, Simon Collett, Daniel Fernandez Ruiz, Linda Earnest, Melissa A. Edeling, Ashley Huey Yiing Yap, Chinn Yi Wong, James P. Cooney, Kathryn C. Davidson, Jason Roberts, Steven Rockman, Bang M. Tran, Julie L. McAuley, Georgia Deliyannis, Samantha L. Grimley, Damian F. J. Purcell, Shafagh A. Waters, Dale I. Godfrey, Dhiraj Hans, Marc Pellegrini, Jason M. Mackenzie, Elizabeth Vincan, William R. Heath and Joseph Torresiadd
Show full author list
remove
Hide full author list
Organoids 2024, 3(1), 18-31; https://doi.org/10.3390/organoids3010002 - 01 Feb 2024
Abstract
►▼
Show Figures
Existing mRNA COVID-19 vaccines have shown efficacy in reducing severe cases and fatalities. However, their effectiveness against infection caused by emerging SARS-CoV-2 variants has waned considerably, necessitating the development of variant vaccines. Ideally, next-generation vaccines will be capable of eliciting broader and more
[...] Read more.
Existing mRNA COVID-19 vaccines have shown efficacy in reducing severe cases and fatalities. However, their effectiveness against infection caused by emerging SARS-CoV-2 variants has waned considerably, necessitating the development of variant vaccines. Ideally, next-generation vaccines will be capable of eliciting broader and more sustained immune responses to effectively counteract new variants. Additionally, in vitro assays that more closely represent virus neutralization in humans would greatly assist in the analysis of protective vaccine-induced antibody responses. Here, we present findings from a SARS-CoV-2 VLP vaccine encompassing three key structural proteins: Spike (S), Envelope (E), and Membrane (M). The VLP vaccine effectively produced neutralizing antibodies as determined by surrogate virus neutralization test, and induced virus-specific T-cell responses: predominantly CD4+, although CD8+ T cell responses were detected. T cell responses were more prominent with vaccine delivered with AddaVax compared to vaccine alone. The adjuvanted vaccine was completely protective against live virus challenge in mice. Furthermore, we utilized air–liquid-interface (ALI)-differentiated human nasal epithelium (HNE) as an in vitro system, which authentically models human SARS-CoV-2 infection and neutralization. We show that immune sera from VLP-vaccinated mice completely neutralized SARS-CoV-2 virus infection, demonstrating the potential of ALI-HNE to assess vaccine induced Nab.
Full article
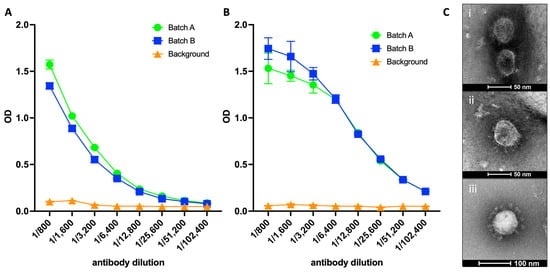
Figure 1
Open AccessFeature PaperArticle
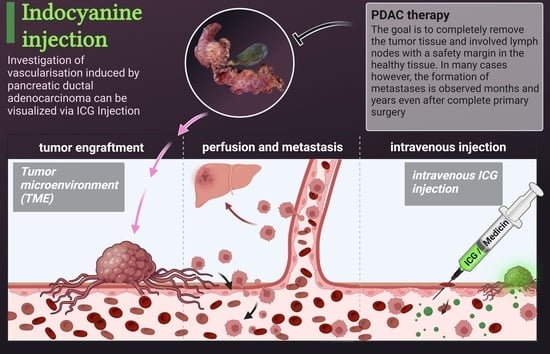
Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing
by
Andreas Ettner-Sitter, Agata Montagner, Jonas Kuenzel, Kathrin Brackmann, Maximilian Schäfer, Robert Schober, Florian Weber, Thiha Aung, Christina Hackl and Silke Haerteis
Organoids 2024, 3(1), 1-17; https://doi.org/10.3390/organoids3010001 - 08 Jan 2024
Cited by 1
Abstract
Although significant improvements have been made in the treatment of pancreatic cancer, its prognosis remains poor with an overall 5-year survival rate of less than 10%. New experimental approaches are necessary to develop novel therapeutics. In this study, the investigation of pancreatic cancer
[...] Read more.
Although significant improvements have been made in the treatment of pancreatic cancer, its prognosis remains poor with an overall 5-year survival rate of less than 10%. New experimental approaches are necessary to develop novel therapeutics. In this study, the investigation of pancreatic cancer tissue growth in the chorioallantoic membrane (CAM) model and the subsequent use of indocyanine green (ICG) injections for the verification of intratumoral perfusion was conducted. ICG was injected into the CAM vasculature to visualize the perfusion of the tumor tissue. The presence of metastasis was investigated through PCR for the human-specific ALU element in the liver of the chicken embryo. Additionally, the usage of cryopreserved pancreatic tumors was established. Intratumoral perfusion of tumor tissue on the CAM was observed in recently obtained and cryopreserved tumors. ALU-PCR detected metastasis in the chick embryos’ livers. After cryopreservation, the tissue was still vital, and the xenografts generated from these tumors resembled the histological features of the primary tumor. This methodology represents the proof of principle for intravenous drug testing of pancreatic cancer in the CAM model. The cryopreserved tumors can be used for testing novel therapeutics and can be integrated into the molecular tumor board, facilitating personalized tumor treatment.
Full article
(This article belongs to the Special Issue Advanced Organoids: New Avenues for Understanding Human Anatomy, Physiology and Development)
►▼
Show Figures
Graphical abstract
Open AccessFeature PaperReview
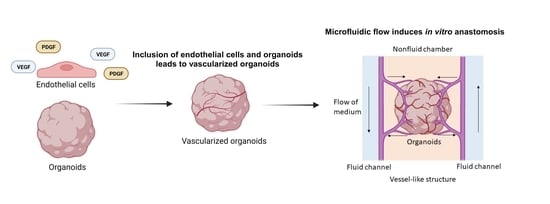
Vascularizing Organoids to Promote Long-Term Organogenesis on a Chip
by
Xinhui Wang, Brent M. Bijonowski and Nicholas A. Kurniawan
Organoids 2023, 2(4), 239-255; https://doi.org/10.3390/organoids2040019 - 07 Dec 2023
Abstract
►▼
Show Figures
Organoids have emerged as a powerful tool for studying organ development, disease modeling, and drug discovery due to their ability to mimic the in vivo structure and function of organs in a three-dimensional in vitro model. During in vivo organ maturation, the process
[...] Read more.
Organoids have emerged as a powerful tool for studying organ development, disease modeling, and drug discovery due to their ability to mimic the in vivo structure and function of organs in a three-dimensional in vitro model. During in vivo organ maturation, the process of vascularization is crucial for the provision of nutrients and oxygen to cells and the removal of waste products as the organ increases in size. Similarly, organoids can grow to sizes greater than the millimeter scale, yet transport of oxygen and nutrients to the center becomes increasingly difficult, often resulting in the formation of a necrotic core. Herein, we provide a concise summary of the recent development of methods to initiate and maintain vascularization of organoids. Broadly, vascularization of organoids has been achieved primarily by two means: generating organoids that contain endothelial cells or employing the secretion of vascular growth factors to promote vascularization. Growth factors play a fundamental role in regulating blood vessel formation through chemical signals that cause changes in the cell–cell adhesions and ultimately the migration of endothelial cells. Furthermore, models with perfusable systems demonstrate that through the application of growth factors and cells, the vascular network in vascularization-based organoids can administer biological substances to the interior of the organoid, opening up new possibilities for long-term organoid culture in vitro. This goal is being realized through the development of bioengineering tools, such as vascularized organoids on a chip, which are currently tested for various organ systems, including the lung, brain, kidney, and tumors, with applications in cancer angiogenesis and metastasis research. Taken together, our review underlines the vast potential of vascularized organoids to improve the understanding of organ development, while also proposing exciting avenues of organoid-on-a-chip and disease modeling.
Full article
Graphical abstract
Open AccessCommentary
Stem Cell-Derived Organoids, Embryoids, and Embryos: Advances in Organismic Development In Vitro Force Us to Re-Focus on Ethical and Legal Aspects of Model Choice
by
Hans-Werner Denker
Organoids 2023, 2(4), 231-238; https://doi.org/10.3390/organoids2040018 - 05 Dec 2023
Abstract
While research on stem cell-derived tissues and organoids is rapidly expanding, the technically related creation of complex embryoids has recently excited a vivid discussion since it raises ethical questions about individuation and the possible gain of viability. The present study focuses on the
[...] Read more.
While research on stem cell-derived tissues and organoids is rapidly expanding, the technically related creation of complex embryoids has recently excited a vivid discussion since it raises ethical questions about individuation and the possible gain of viability. The present study focuses on the onset of organismic development and the proposed biological and legal definitions for the terms embryo, embryoid, and organoid. It is concluded that such considerations have become important for investigators’ choices of the appropriate in vitro model systems, allowing the formation of organoids vs. complex embryoids.
Full article
Open AccessReview
Modelling Meningioma Using Organoids: A Review of Methodologies and Applications
by
Clara Elena López Vásquez, Clint Gray, Claire Henry and Matthew J. Munro
Organoids 2023, 2(4), 218-230; https://doi.org/10.3390/organoids2040017 - 04 Dec 2023
Abstract
Meningiomas are the most common tumours of the central nervous system. According to the World Health Organization (WHO), this disease is classified into three different grades: 80% of meningioma patients present with benign grade I tumours, while less than 2% present with malignant
[...] Read more.
Meningiomas are the most common tumours of the central nervous system. According to the World Health Organization (WHO), this disease is classified into three different grades: 80% of meningioma patients present with benign grade I tumours, while less than 2% present with malignant grade III meningiomas. Despite affecting thousands of people worldwide, much remains unknown about this disease, and the development of systemic treatments is still far behind in comparison to other types of tumours. Therefore, forming 3D structures (spheroids and organoids) could facilitate research on the mechanisms of formation, proliferation, migration, and invasion of these, for the most part, benign tumours, while also helping in the process of drug development. To date, there are three published methods for the formation of meningioma organoids primarily derived from patient tissue samples. Organoids offer many advantages in the development of treatments because they recapitulate the cellular complexity within tumours. These new methodological advances could open a substantial number of possibilities for the further characterisation and treatment of meningiomas. This review includes an overview of the disease and a description and comparison of established protocols for meningioma organoid formation.
Full article
Open AccessArticle
The Rapid Generation of Cell-Laden, FACS-Compatible Collagen Gels
by
Yi Xiao, Qiaoling Huang, Jesse W. Collins, Julie Brouchon, Jeffery A. Nelson, Zachary Niziolek, Alison O’Neil, Fangfu Ye, David A. Weitz and John A. Heyman
Organoids 2023, 2(4), 204-217; https://doi.org/10.3390/organoids2040016 - 17 Nov 2023
Abstract
►▼
Show Figures
A three-dimensional cell culture in hydrogel beads can support cell growth and differentiation into multi-cellular structures, and these gel beads could be used as building blocks for more complex three-dimensional assemblies. This requires hydrogel beads that are robust enough to sort via FACS
[...] Read more.
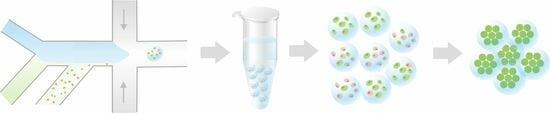
A three-dimensional cell culture in hydrogel beads can support cell growth and differentiation into multi-cellular structures, and these gel beads could be used as building blocks for more complex three-dimensional assemblies. This requires hydrogel beads that are robust enough to sort via FACS yet can be degraded by cell-secreted enzymes. Collagen polymers form hydrogels that are excellent cell growth substrates; however, collagen-containing hydrogel beads typically include additional polymers that limit their degradation. Here, we introduce a simple microfluidic method to generate robust, sortable, cell-laden collagen hydrogel beads. We use on-device pH control to trigger collagen gelation without exposing cells to low pH, ensuring high cell viability. We fabricate microfluidic devices to generate droplets with a wide size range, as demonstrated by production of both small (~55 µm diameter) and large (~300 µm diameter) collagen gels. All hydrogels are sufficiently robust to allow for sorting using FACS. Moreover, high cell viability is maintained throughout the process.
Full article
Graphical abstract
Open AccessArticle
SMAD1 Is Dispensable for CDX2 Induction but Required for the Repression of Ectopic Small-Intestinal Gene Expression in Human-Pluripotent-Stem-Cell-Derived Colonic Organoids
by
Na Qu, Abdelkader Daoud, Braxton Jeffcoat and Jorge O. Múnera
Organoids 2023, 2(4), 192-203; https://doi.org/10.3390/organoids2040015 - 14 Nov 2023
Abstract
The generation of gastrointestinal tissues from human pluripotent stem cells has provided unprecedented insight into the molecular mechanisms that drive the patterning of the primitive gut tube. Previous work has identified bone-morphogenetic-protein (BMP) signaling as an important mediator of mid/hindgut versus foregut and
[...] Read more.
The generation of gastrointestinal tissues from human pluripotent stem cells has provided unprecedented insight into the molecular mechanisms that drive the patterning of the primitive gut tube. Previous work has identified bone-morphogenetic-protein (BMP) signaling as an important mediator of mid/hindgut versus foregut and hindgut versus midgut cell fate choice. Inhibition of BMP signaling during gut tube morphogenesis inhibits the expression of the pan-intestinal transcription factor CDX2. Treatment of CDX2+ mid/hindgut cultures with BMP patterns them into hindgut, which gives rise to colonic organoids (HCOs). While the role for BMP signaling is clear, the molecular mechanisms through which BMP signaling patterns the mid/hindgut and colon remain unclear. BMPs bind to BMP receptors, activating a signaling cascade that results in the activation of SMADs, which function as transcription factors. We hypothesized that one of these factors, SMAD1, would be necessary for establishing the CDX2 domain and the colon domain. Unexpectedly, endoderm derived from SMAD1-deficient induced pluripotent stem cells was capable of inducing CDX2 in response to WNT and FGF signaling. In addition, CDX2+ gut tube cultures could activate posterior HOX genes in response to BMP. However, examination of HCOs following cytodifferentiation revealed that SMAD1-deficient HCOs ectopically expressed small-intestinal markers despite expressing posterior HOX genes. These results indicate that there is redundancy of SMADs during early hindgut patterning but that SMAD1 is required for the inhibition of small-intestinal gene expression in HCOs.
Full article
(This article belongs to the Special Issue Intestinal Organoid)
►▼
Show Figures
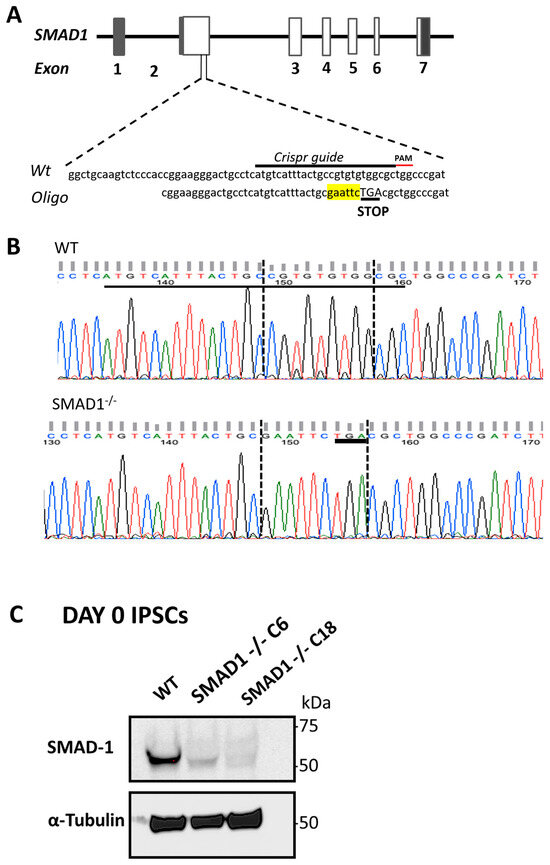
Figure 1
Open AccessArticle
Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods
by
Remi Yokoi, Nami Nagafuku, Yuto Ishibashi, Naoki Matsuda and Ikuro Suzuki
Organoids 2023, 2(4), 177-191; https://doi.org/10.3390/organoids2040014 - 26 Oct 2023
Abstract
►▼
Show Figures
Ensuring drug safety for patients with specific neurological disorders is of paramount importance. For instance, certain antiepileptic drugs (AEDs) are contraindicated in Dravet Syndrome (DS), which is characterized by a deficiency in Na+ channel function. Constructing in vitro assessment methods capable of
[...] Read more.
Ensuring drug safety for patients with specific neurological disorders is of paramount importance. For instance, certain antiepileptic drugs (AEDs) are contraindicated in Dravet Syndrome (DS), which is characterized by a deficiency in Na+ channel function. Constructing in vitro assessment methods capable of detecting contraindicated drug responses and medication effects on neurons derived from DS patients is highly anticipated for drug safety assessment and therapeutic innovation. This study used micro electrode array (MEA) measurements with low-frequency analysis on human iPSC-derived DS organoids to investigate AED responses. When exposed to the contraindicated drugs carbamazepine and phenytoin, the number of network oscillations increased in DS organoids while maintaining oscillation intensity. Furthermore, carbamazepine administration appeared to enhance activities beyond oscillations which is partially consistent with findings in the DS mouse model. Conversely, treatment with the therapeutic drug sodium valproate resulted in a similar decrease in activity both in healthy and DS organoids. The frequency characteristics of spontaneous firings and AEDs responsiveness in DS organoids demonstrated partial correlation with typical electroencephalography patterns observed in vivo. In conclusion, this study, employing MEA measurements with low-frequency analysis, revealed contraindicated drug responses and disease-specific functional characteristics in DS organoids, effective for DS patient safety assessment, precision medicine, and antiepileptic drug screening.
Full article
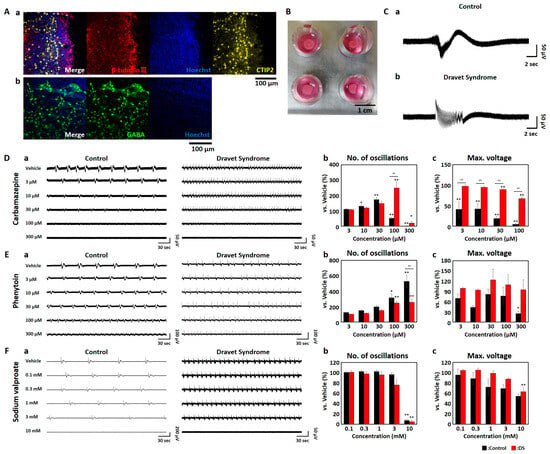
Figure 1
Open AccessCommunication
Matrigel Tunes H9 Stem Cell-Derived Human Cerebral Organoid Development
by
R. Chris Estridge, Jennifer E. O’Neill and Albert J. Keung
Organoids 2023, 2(4), 165-176; https://doi.org/10.3390/organoids2040013 - 05 Oct 2023
Abstract
►▼
Show Figures
Human cerebral organoids are readily generated from human embryonic stem cells and human induced pluripotent stem cells and are useful in studying human neurodevelopment. Recent work with human cerebral organoids have explored the creation of different brain regions and the impacts of soluble
[...] Read more.
Human cerebral organoids are readily generated from human embryonic stem cells and human induced pluripotent stem cells and are useful in studying human neurodevelopment. Recent work with human cerebral organoids have explored the creation of different brain regions and the impacts of soluble and mechanical cues. Matrigel is a gelatinous, heterogenous mixture of extracellular matrix proteins, morphogens, and growth factors secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. It is a core component of almost all cerebral organoid protocols, generally supporting neuroepithelial budding and tissue polarization; yet, its roles and effects beyond its general requirement in organoid protocols are not well understood, and its mode of delivery is variable, including the embedding of organoids within it or its delivery in soluble form. Given its widespread usage, we asked how H9 stem cell-derived hCO development and composition are affected by Matrigel dosage and delivery method. We found Matrigel exposure influences organoid size, morphology, and cell type composition. We also showed that greater amounts of Matrigel promote an increase in the number of choroid plexus (ChP) cells, and this increase is regulated by the BMP4 pathway. These results illuminate the effects of Matrigel on human cerebral organoid development and the importance of delivery mode and amount on organoid phenotype and composition.
Full article
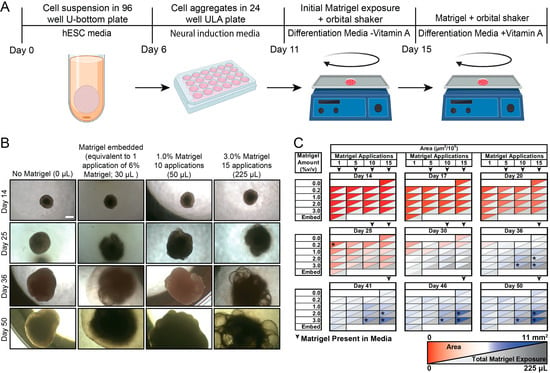
Figure 1
Open AccessCommunication
The Intricacies of Inflammatory Bowel Disease: A Preliminary Study of Redox Biology in Intestinal Organoids
by
Georg Csukovich, Janina Huainig, Selina Troester, Barbara Pratscher and Iwan Anton Burgener
Organoids 2023, 2(3), 156-164; https://doi.org/10.3390/organoids2030012 - 03 Sep 2023
Cited by 1
Abstract
We evaluated the redox status, precisely glutathione levels, which have a major impact in cellular detoxification and antioxidant defence in IBD-derived and healthy intestinal organoids. Therefore, we wanted to explore the differences in terms of their redox balance and mitochondrial fitness. To this
[...] Read more.
We evaluated the redox status, precisely glutathione levels, which have a major impact in cellular detoxification and antioxidant defence in IBD-derived and healthy intestinal organoids. Therefore, we wanted to explore the differences in terms of their redox balance and mitochondrial fitness. To this end, we introduced a Grx1-roGFP2 construct into the organoids by lentiviral transduction before performing a stress assay by treating the organoids with hydrogen peroxide and examined the GSH/GSSG ratio using confocal imaging. Using ratio imaging, we could detect statistically significant differences between healthy and IBD-derived samples. To gain more insight, we also performed a GSH/GSSG assay, which directly measured glutathione levels. This analysis revealed that both organoid lines had higher levels of oxidized glutathione due to the stress treatment demonstrated by a lower GSH/GSSG ratio compared to the untreated control. Nevertheless, the results showed no significant difference between healthy and IBD-derived organoids. We further challenged organoids with hydrogen peroxide after incubation with MitoTracker® to see if mitochondrial fitness might be different in IBD-derived organoids. However, these results were also very comparable. In summary, our preliminary findings indicate that both organoid lines demonstrate a well-functioning system in terms of analysis but show no clear difference between healthy and IBD-derived samples.
Full article
(This article belongs to the Special Issue Intestinal Organoid)
►▼
Show Figures
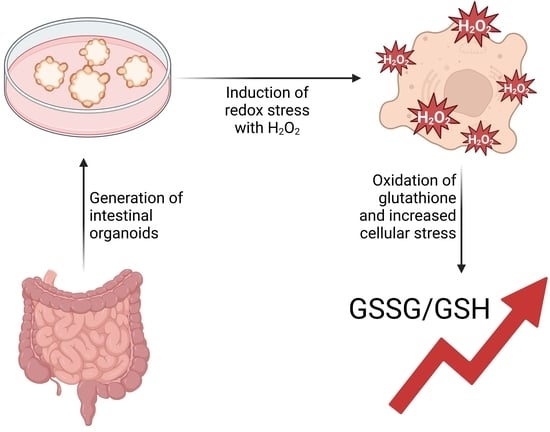
Graphical abstract
Open AccessReview
Incorporating Immune Cells into Organoid Models: Essential for Studying Human Disease
by
Ania Bogoslowski, Meilin An and Josef M. Penninger
Organoids 2023, 2(3), 140-155; https://doi.org/10.3390/organoids2030011 - 12 Aug 2023
Cited by 2
Abstract
►▼
Show Figures
Organoid-based research has made significant discoveries and contributions to our understanding of human organ function in both health and disease. To continue making progress, it is crucial to acknowledge the crucial role of the immune system in all organs. Various immune cells, such
[...] Read more.
Organoid-based research has made significant discoveries and contributions to our understanding of human organ function in both health and disease. To continue making progress, it is crucial to acknowledge the crucial role of the immune system in all organs. Various immune cells, such as macrophages, T cells, and neutrophils, are resident in almost all human tissues and play essential roles in organ homeostasis, function, and disease. Using diverse methods, researchers have begun integrating immune cells into organoid models, leading to more physiologically relevant models that better represent various aspects of human disease. These methods range from immune cell injection to co-culture and tissue expansion with existing immune cells. Immune cells can be sourced from mature patients or generated from stem cells as immature immune cells. The successful incorporation of immune cells into organoids will enhance our understanding of organ function and provide a more accurate approximation of human disease.
Full article
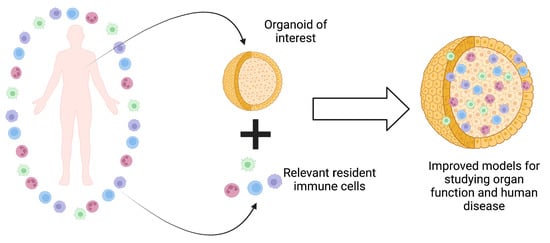
Graphical abstract
Open AccessReview
Organoid Models and Next-Generation Sequencing for Bone Marrow and Related Disorders
by
Magdalena Rausch, Neelam Iqbal, Shelly Pathak, Heather E. Owston and Payal Ganguly
Organoids 2023, 2(3), 123-139; https://doi.org/10.3390/organoids2030010 - 01 Jul 2023
Cited by 3
Abstract
►▼
Show Figures
Challenges to the musculoskeletal system negatively impact the quality of life of people suffering from them, leading to pain, a decline in mobility, genetic alterations, and potential disorders. The bone marrow (BM) forms an integral part of the musculoskeletal system responsible for erythropoiesis
[...] Read more.
Challenges to the musculoskeletal system negatively impact the quality of life of people suffering from them, leading to pain, a decline in mobility, genetic alterations, and potential disorders. The bone marrow (BM) forms an integral part of the musculoskeletal system responsible for erythropoiesis and optimal survival of the various immune and stem cells within the BM. However, due to its dynamic and complex three-dimensional (3D) structure, replicating the BM physiologically in traditional two-dimensional (2D) cell culture settings is often challenging, giving rise to the need for 3D in vitro models to better dissect the BM and its regeneration. Several researchers globally have been investigating various approaches to define an appropriate 3D model for their research. Organoids are novel preclinical models that provide a 3D platform for several tissues and have been analysed using next-generation sequencing (NGS) to identify new molecular pathways at the genetic level. The 3D in vitro models and organoids are increasingly considered important platforms for precision medicine. This review outlines the current knowledge of organoid and 3D in vitro models for the BM. We also discuss different types of 3D models which may be more adaptable for the BM. Finally, we critically review the NGS techniques used for such models and the future combination of these techniques.
Full article
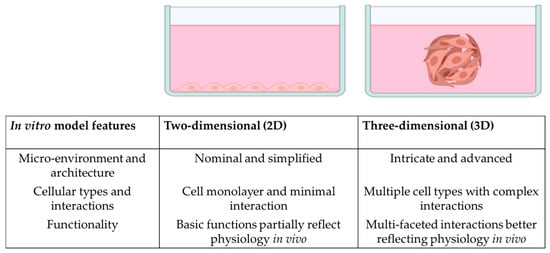
Figure 1
Open AccessEditorial
Organoids Are Us
by
Elizabeth Vincan
Organoids 2023, 2(2), 120-122; https://doi.org/10.3390/organoids2020009 - 16 Jun 2023
Abstract
“Organoids Are Us” is an annual one-day symposium organised to highlight the advances in science and medicine that are the direct result of organoid technology [...]
Full article
(This article belongs to the Special Issue Advances in Organoid Technology—Selected Papers from "Organoids Are Us 2022")
Open AccessArticle
A Microwell Device for the Efficient Generation of Arrays of Microtissues and Humanized Bone Marrow Micro-Ossicles
by
Kathryn Futrega, Md. Shafiullah Shajib, Pamela G. Robey and Michael R. Doran
Organoids 2023, 2(2), 102-119; https://doi.org/10.3390/organoids2020008 - 01 Jun 2023
Abstract
(1) Background: There are no high-throughput microtissue platforms for generating bone marrow micro-ossicles. Herein, we describe a method for the assembly of arrays of microtissues from bone marrow stromal cells (BMSC) in vitro and their maturation into bone marrow micro-ossicles in vivo. (2)
[...] Read more.
(1) Background: There are no high-throughput microtissue platforms for generating bone marrow micro-ossicles. Herein, we describe a method for the assembly of arrays of microtissues from bone marrow stromal cells (BMSC) in vitro and their maturation into bone marrow micro-ossicles in vivo. (2) Methods: Discs with arrays of 50 microwells were used to assemble microtissues from 3 × 105 BMSCs each on a nylon mesh carrier. Microtissues were cultured in chondrogenic induction medium followed by hypertrophic medium in an attempt to drive endochondral ossification, and then they were implanted in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice, where they were remodeled into bone marrow micro-ossicles. Mice were transplanted with 105 human umbilical cord blood CD34+ cells. (3) Results: Micro-ossicles contained more human CD45+ cells, but fewer human CD34+ progenitor cells than mouse marrow. Human hematopoietic progenitor cells cycle rapidly at non-physiological rates in mouse marrow, and reduced CD34+ cell content in micro-ossicles is consistent with the notion that the humanized niche better controls progenitor cell cycling. (4) Conclusions: Assembling microtissues in microwells, linked by a nylon membrane carrier, provides an elegant method to manufacture and handle arrays of microtissues with bone organ-like properties. More generally, this approach and platform could aid bridging the gap between in vitro microtissue manipulation and in vivo microtissue implantation.
Full article
(This article belongs to the Special Issue Organoids and Advanced 3D Models in Biomedical Research)
►▼
Show Figures
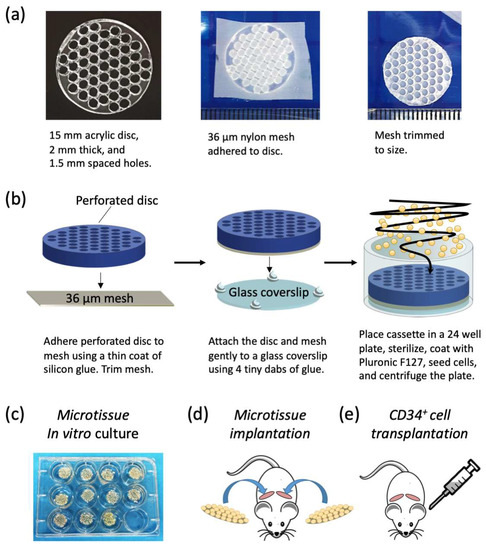
Figure 1
Open AccessFeature PaperArticle
Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease
by
Pin Shie Quah, Bang M. Tran, Vincent D.A. Corbin, Jessie J.-Y. Chang, Chinn Yi Wong, Andrés Diaz-Méndez, Carol A. Hartley, Weiguang Zeng, Eric Hanssen, Zlatan Trifunovic, Patrick C. Reading, David C. Jackson, Elizabeth Vincan, Lachlan J.M. Coin and Georgia Deliyannis
Organoids 2023, 2(2), 82-101; https://doi.org/10.3390/organoids2020007 - 11 May 2023
Cited by 1
Abstract
Bovine respiratory disease (BRD) is the leading cause of morbidity and mortality in feedlot cattle. Bovine herpesvirus-1 (BHV-1) is one of the main culprits of BRD; however, research on BHV-1 is hampered by the lack of suitable models for infection and drug testing.
[...] Read more.
Bovine respiratory disease (BRD) is the leading cause of morbidity and mortality in feedlot cattle. Bovine herpesvirus-1 (BHV-1) is one of the main culprits of BRD; however, research on BHV-1 is hampered by the lack of suitable models for infection and drug testing. In this study, we established a novel bovine tracheal organoid culture grown in a basement membrane extract type 2 (BME2) matrix and compared it with the air–liquid interface (ALI) culture system. After differentiation, the matrix-embedded organoids developed beating cilia and demonstrated a transcriptomic profile similar to the ALI culture system. The matrix-embedded organoids were also highly susceptible to BHV-1 infection and immune stimulation by Pam2Cys, an immunomodulator, which resulted in robust cytokine production and tracheal antimicrobial peptide mRNA upregulation. However, treatment of bovine tracheal organoid cultures with Pam2Cys was not sufficient to inhibit viral infection or replication, suggesting a role of the non-epithelial cellular microenvironment in vivo.
Full article
(This article belongs to the Special Issue Advances in Organoid Technology—Selected Papers from "Organoids Are Us 2022")
►▼
Show Figures

Graphical abstract
Open AccessEditorial
“Organoids”: Insights from the First Issues
by
Philipp Wörsdörfer and Süleyman Ergün
Organoids 2023, 2(2), 79-81; https://doi.org/10.3390/organoids2020006 - 07 Apr 2023
Abstract
Organoids are taking the scientific world by storm, revolutionizing the ways in which we study complex biological systems [...]
Full article
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biomolecules, Cancers, Cells, IJMS, JCM, JMP, Organoids
Novel Discoveries in Oncology
Topic Editors: Alessia Filippone, Giovanna Casili, Marika Lanza, Michela CampoloDeadline: 30 November 2024

Conferences
Special Issues
Special Issue in
Organoids
The Current Applications and Potential of Stem Cell-Derived Organoids
Guest Editors: James Adjaye, Nina GraffmannDeadline: 31 May 2024
Special Issue in
Organoids
Organoids and Cancer Models
Guest Editor: Xuefeng LiuDeadline: 30 June 2024
Special Issue in
Organoids
Organoids and Metabolic Diseases
Guest Editor: Cyril CorbetDeadline: 31 December 2024