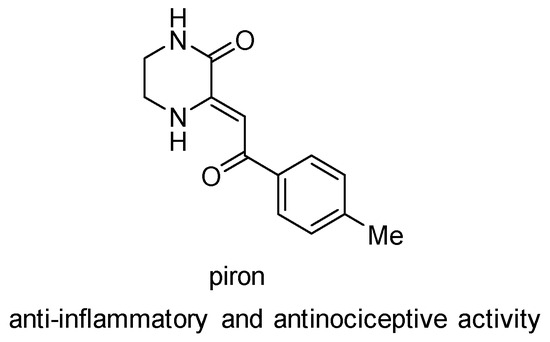
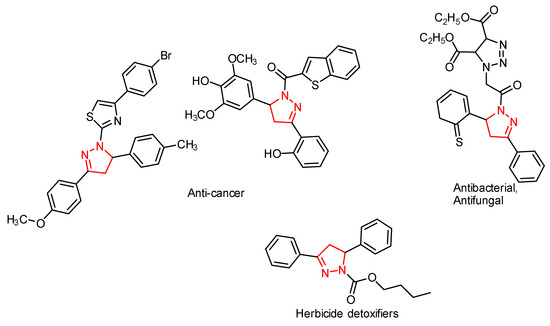
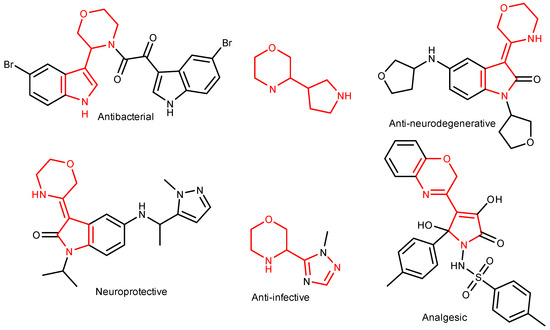
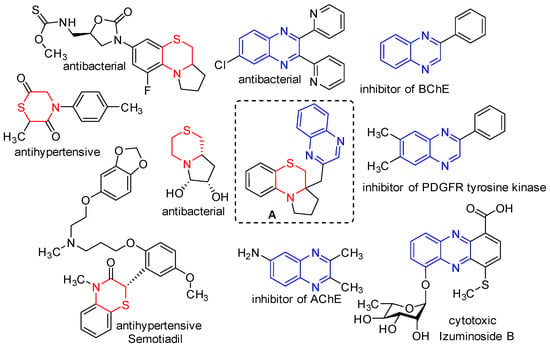
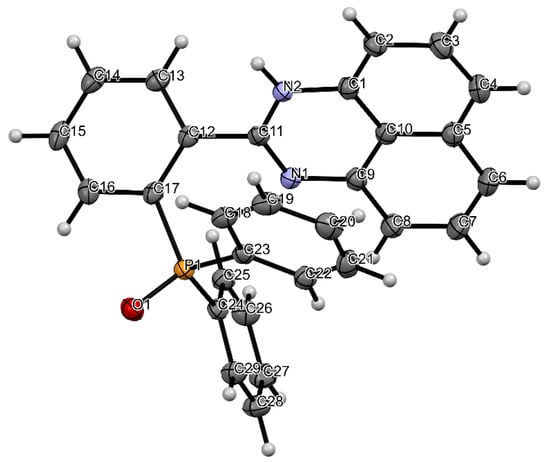
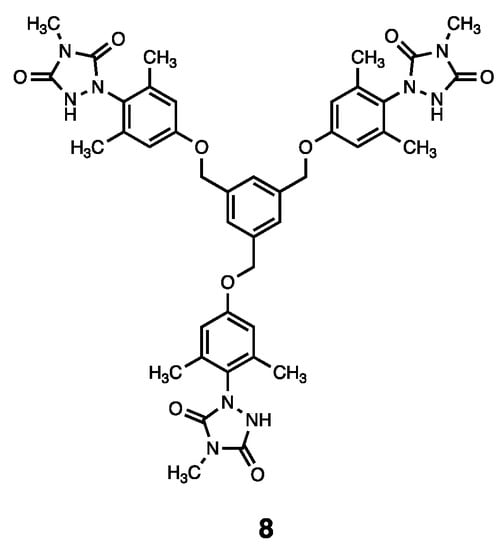
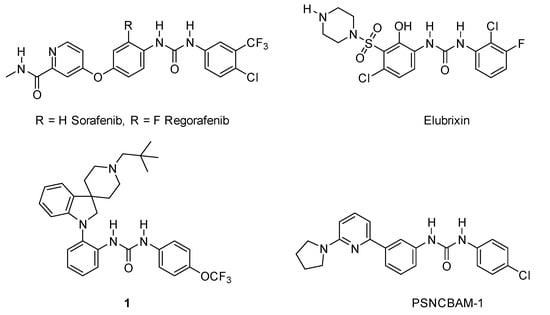
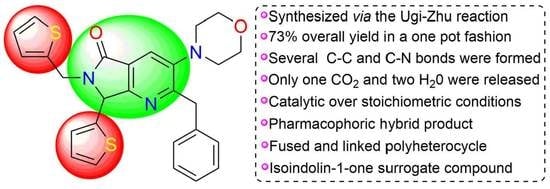
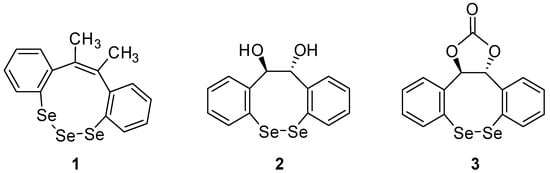
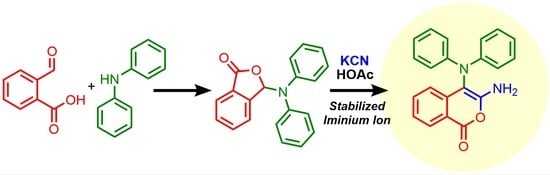
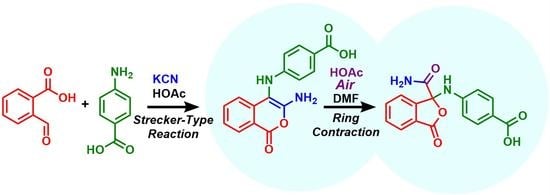
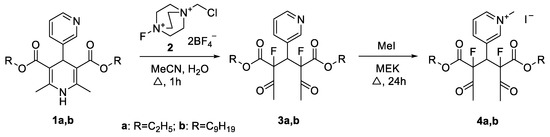
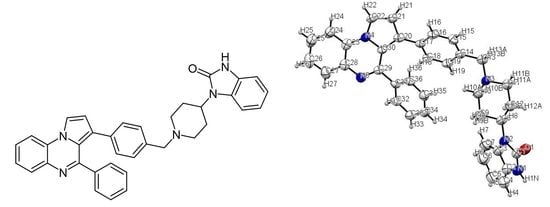
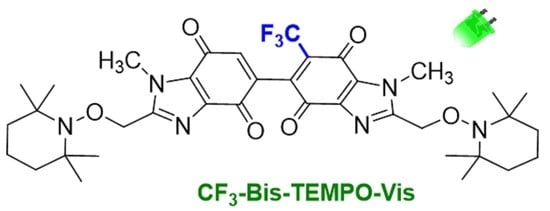
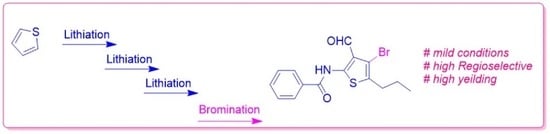
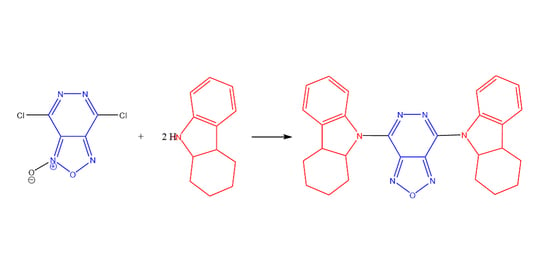
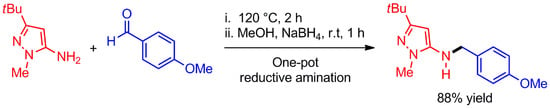
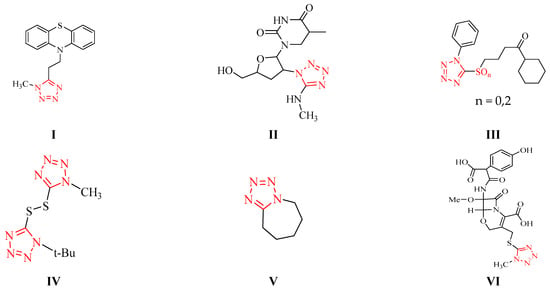
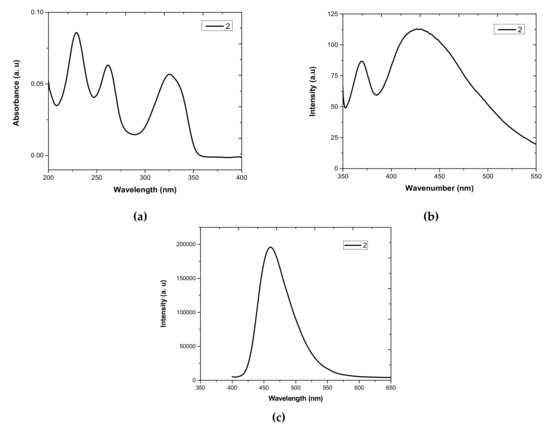
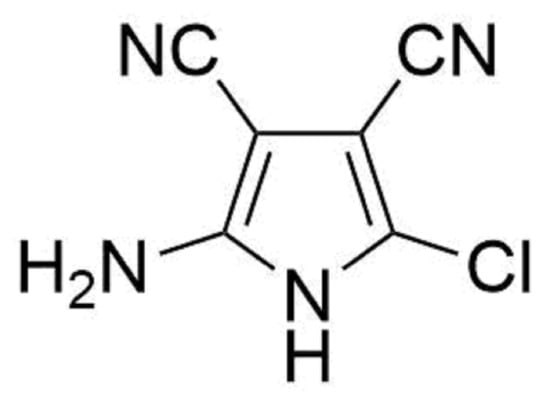
Heterocycle Reactions
A topical collection in Molbank (ISSN 1422-8599). This collection belongs to the section "Organic Synthesis".
Viewed by 142709Editor
Interests: free radical organic and polymer chemistry; heterocyclic and medicinal chemistry
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
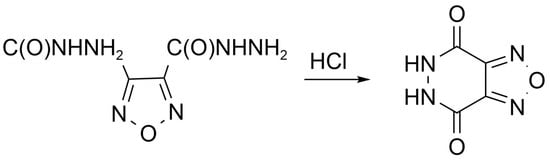
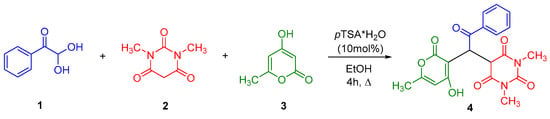
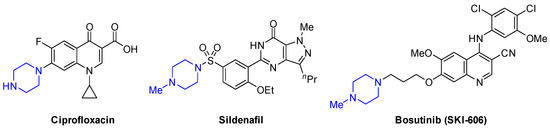
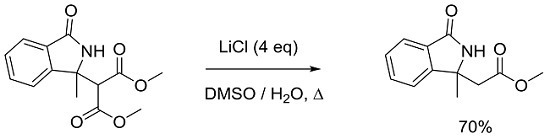
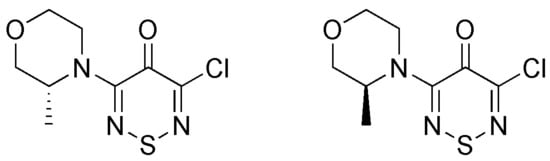
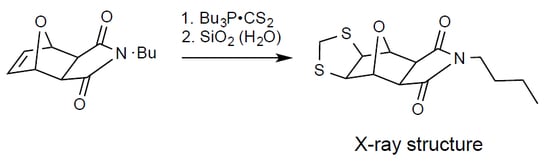
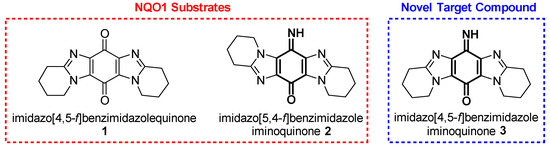
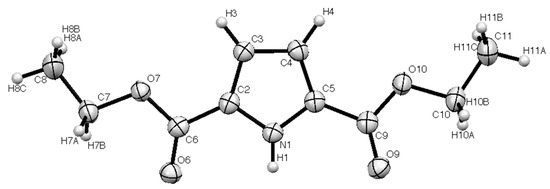
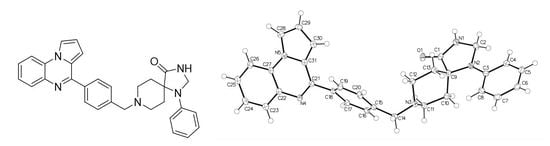
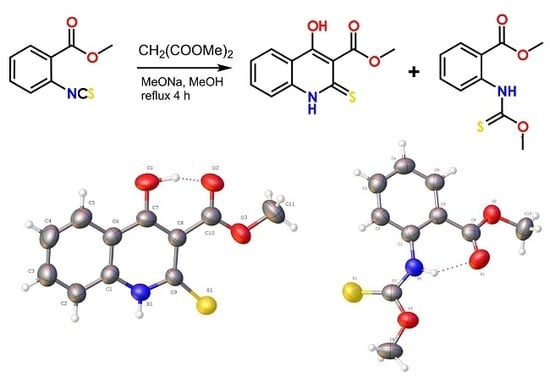
According to "Heterocyclic Chemistry" by J. A. Joule and K. Mills (Wiley, 5th Ed), "heterocyclic chemistry comprises at least half of all organic chemistry research worldwide". Many reactions proceed as predicted, while other reactions give unusual products, which perhaps do not fit the intended use but are nevertheless chemically interesting. Some reactions give more than one product, with selectivity tunable through optimization or biasing of reaction conditions. Often, interesting "side-products" are neglected in pursuit of a target compound.
This Special Issue welcomes the submission of any suitably fully characterized novel compound(s) from a given heterocyclic compound reaction. Please note that the reaction product does not necessarily have to be heterocyclic.
Prof. Dr. Fawaz Aldabbagh
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molbank is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 500 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Heterocyclic chemistry
- Aromatic heterocycles
- Non-aromatic heterocycles
- Fused heterocycles
- Nitrogen
- Oxygen
- Sulfur
- Radical
- Spectroscopic properties
- Halogenation
- Organic chemistry
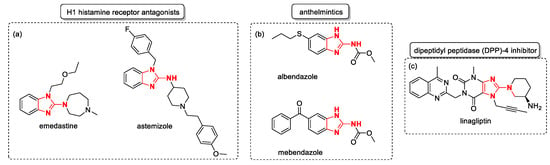
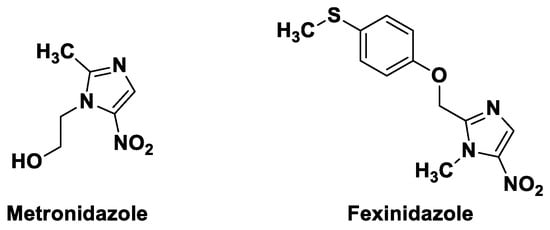
- Medicinal chemistry