Bioleaching (Closed)
A topical collection in Minerals (ISSN 2075-163X). This collection belongs to the section "Mineral Processing and Extractive Metallurgy".
Viewed by 120551Editors
Interests: biocorrosion; bioelectrochemistry; bioflotation; biogeochemistry; bioleaching; biomining; biooxidation; bioprecipitation; bioreduction; bioremediation; circular economy; resource recovery; waste management; wastewater treatment
Special Issues, Collections and Topics in MDPI journals
Interests: geomicrobiology; biohydrometallurgy; biogeochemical processes; biomining; bioleaching; mineral-microbe interactions
Interests: biohydrometallurgy; bioleaching; biomining; mineral-microbe interactions; biorecovery; biomaterials; bioremediation
2. Department of Hydraulic and Environmental Engineering, School of Engineering, Pontificia Universidad Católica de Chile, Santiago 8320000, Chile
Interests: biofilm formation of bioleaching microorganisms; fluorescence microscopy, massive image analyses and OMICS techniques; microbial genetics and extracellular polymeric substances analysis and characterization; characterization of interactions and cell–cell communication in bioleaching consortia; changes in microbial diversity during bioleaching of chalcopyrite-containing ores; EPS production and analysis and development of methodologies for massive image analysis of biofilms of axenic and mixed bioleaching microbial consortia on pyrite and chalcopyrite surfaces
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
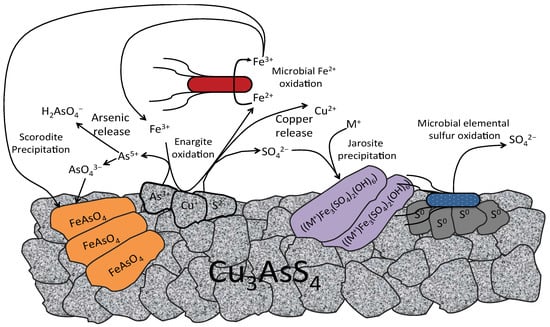
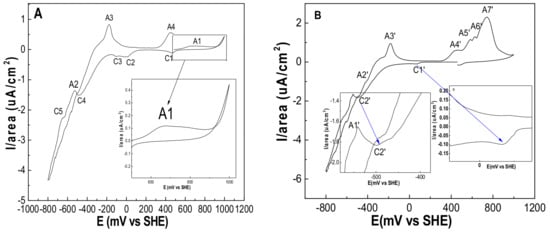
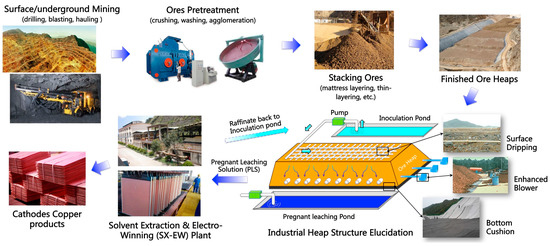
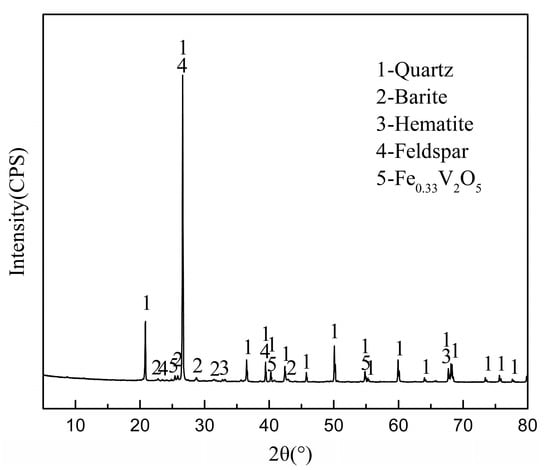
Since the discovery of bioleaching microorganisms and their role in metal extraction in the 1940s, a number of approaches have been developed to enhance microbially catalysed solubilisation of metals. These include reactor/tank, vat, lagoon, heap, dump, in place or in situ leaching techniques. Bioleaching has enabled the transformation of uneconomic resources to reserves, and thus helped to alleviate the challenges related to continually declining ore grades. Commercial biomining applications have mainly targeted copper, gold, uranium, nickel, cobalt and zinc sulfides. More recently, the possibilities of bioleaching oxide ores and extracting other commodities such as rare earth elements and phosphorus have also been explored. Progress in characterising microbial strains and communities has increased our understanding of the microbial catalysts, and facilitated the optimisation of bioleaching processes. For this topical collection, we invite contributions on various aspects of bioleaching, including but not limited to bioleaching methods, mechanisms, microorganisms, and applications to extract various commodities from ores, concentrates as well as waste materials.
Dr. Anna H. Kaksonen
Prof. Dr. Sabrina Hedrich
Dr. Elaine Govender-Opitz
Dr. Mario Vera Véliz
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Minerals is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- biohydrometallurgy
- bioleaching
- bioleaching microorganisms
- bioleaching methods
- bioleaching mechanisms
- bioleaching of ores and concentrates
- bioleaching of waste materials
- biomining
- biooxidation
- biorecovery
- oxidative bioleaching
- reductive bioleaching
Related Special Issue
- Advances in Biohydrometallurgy in Minerals (6 articles)