-
 Trade-Off between Enteric and Manure Methane Emissions Linked with Bacterial Ecology in Dairy Cows Fed Contrasting Diets
Trade-Off between Enteric and Manure Methane Emissions Linked with Bacterial Ecology in Dairy Cows Fed Contrasting Diets -
 Dietary Chemical Composition and Enteric Methane Production: Formulating Cattle Diets to Decrease Emissions
Dietary Chemical Composition and Enteric Methane Production: Formulating Cattle Diets to Decrease Emissions -
 Energy Security Blind Spots of Gas, Oil, and Coal Exporters
Energy Security Blind Spots of Gas, Oil, and Coal Exporters -
 Methane Biofiltration Processes: A Summary of Biotic and Abiotic Factors
Methane Biofiltration Processes: A Summary of Biotic and Abiotic Factors
Journal Description
Methane
Methane
is an international, peer-reviewed, open access journal on all aspects of methane published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 17.3 days after submission; acceptance to publication is undertaken in 9.7 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
Fungal Methane Production Controlled by Oxygen Levels and Temperature
Methane 2024, 3(2), 257-275; https://doi.org/10.3390/methane3020015 - 19 Apr 2024
Abstract
►
Show Figures
Saprotrophic fungi, key players in global carbon cycling, have been identified as methane (CH4) sources not yet accounted for in the global CH4 budget. This study, for the first time, explores the influence of oxygen (O2) and temperature
[...] Read more.
Saprotrophic fungi, key players in global carbon cycling, have been identified as methane (CH4) sources not yet accounted for in the global CH4 budget. This study, for the first time, explores the influence of oxygen (O2) and temperature on CH4 production by two fungi, Laetiporus sulphureus and Pleurotus sapidus. To explore the relationship between these parameters and fungal CH4 formation, we examined CH4 formation under varying O2 levels (0 to 98%) and temperatures (17, 27, and 40 °C) during fungal growth on pine wood, beech wood, and grass under sterile conditions. Our findings show that fungal CH4 formation strongly depends on O2 levels. Methane formation was highest when O2 levels exceeded 5%, whilst no CH4 formation was observed after complete O2 consumption. Reintroducing O2 immediately resumed fungal CH4 production. Methane formation normalized to O2 consumption (CH4_norm) showed a different pattern. L. sulphureus showed higher CH4_norm rates with higher O2 levels, whereas P. sapidus showed elevated rates between 0 and 5%. Temperature also significantly influenced CH4 and CH4_norm rates, with the highest production at 27 °C, and comparatively lower rates at 17 and 40 °C. These findings demonstrate the importance of O2 levels and temperature in fungal CH4 emissions, which are essential for refining CH4 source predictions.
Full article
Open AccessReview
A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance
by
Xiaoyong Li, Zhi Wang, Yun He, Yuzhong Wang, Shilei Wang, Zehui Zheng, Songtao Wang, Jingliang Xu, Yafan Cai and Hanjie Ying
Methane 2024, 3(2), 227-256; https://doi.org/10.3390/methane3020014 - 15 Apr 2024
Abstract
►▼
Show Figures
Low and unstable digestion performance is a challenging issue for anaerobic digestion, which prompts researchers to develop new strategies. In addition to traditional approaches such as co-digestion, pre-treatment, and recirculation, some emerging strategies, namely additive processes and microaeration, have also been recognized and
[...] Read more.
Low and unstable digestion performance is a challenging issue for anaerobic digestion, which prompts researchers to develop new strategies. In addition to traditional approaches such as co-digestion, pre-treatment, and recirculation, some emerging strategies, namely additive processes and microaeration, have also been recognized and developed in recent years. Many studies have evaluated the effect of these strategies on digestion performance. However, their comprehensive analysis is lacking, especially regarding the mechanisms of the different strategies. This review presents a comprehensive overview of research progress on these strategies based on the latest research, considering the five main strategies listed above. Through critical thinking, a summary of their mechanism, reactor performance, and availability of these strategies is presented. The results demonstrate that the contribution of microaeration is mainly to balance the composition and activity of hydrolysis, acidogenesis, and methanogenic archaea. Recirculation and co-digestion mainly balance mass and reaction environments. Pre-treatment, such as removing lignin, reducing cellulose crystallinity, and increasing the substrate-specific surface area, makes the characteristics of the substrate more conducive to the digestion of microorganisms. The mechanism of additive strategies varies greatly depending on the type of additive, such as enhancing interspecies electron transfer through conductive materials, resisting adverse digestion conditions through functional microbial additives, and accelerating nutrient absorption by regulating the bioavailability of trace elements. Although these strategies have different mechanisms for promoting digestion performance, their ultimate effect is to allow the parameters of the reactor to reach an ideal status and then achieve a balance among the substance, microorganisms, and water in an anaerobic reactor.
Full article
Figure 1
Open AccessArticle
Thermochemical Pretreatment for Improving the Psychrophilic Anaerobic Digestion of Coffee Husks
by
Tzyy Shyuan Yang, Carla Flores-Rodriguez, Lorena Torres-Albarracin and Ariovaldo José da Silva
Methane 2024, 3(2), 214-226; https://doi.org/10.3390/methane3020013 - 29 Mar 2024
Abstract
Psychrophilic anaerobic digestion emerges as an appealing integrated solution for the management of agricultural waste, particularly for farmers in regions where the average temperature does not exceed 26 °C, as seen in coffee cultivation. Therefore, this study seeks to assess the biomethane potential
[...] Read more.
Psychrophilic anaerobic digestion emerges as an appealing integrated solution for the management of agricultural waste, particularly for farmers in regions where the average temperature does not exceed 26 °C, as seen in coffee cultivation. Therefore, this study seeks to assess the biomethane potential of thermochemical-treated coffee husk through psychrophilic anaerobic digestion (C3-20 °C-w/pretreatment). To examine its viability, outcomes were compared with reactors operating at both mesophilic (C1-35 °C) and psychrophilic (C2-20 °C) conditions, albeit without the use of pretreated coffee husk. The C3-20 °C-w/pretreatment test demonstrated a 36.89% increase (150.47 NmL CH4/g VS; 161.04 NmL CH4/g COD), while the C1-35 °C test exhibited a 24.03% increase (124.99 NmL CH4/g VS; 133.77 NmL CH4/g COD), both in comparison to the C2-20 °C test (94.96 NmL CH4/g VS; 101.63 NmL CH4/g COD). Notably, the C3-20 °C-w/pretreatment trial yielded superior outcomes, accompanied by an associated energy output of 3199.25 GWh/year, sufficient to meet the annual energy demands of 494 residences. This marks an increase of 83 and 182 million residences compared to the mesophilic and psychrophilic AD of CH without pretreatment, respectively.
Full article
(This article belongs to the Special Issue Anaerobic Digestion Process: Converting Waste to Energy)
►▼
Show Figures
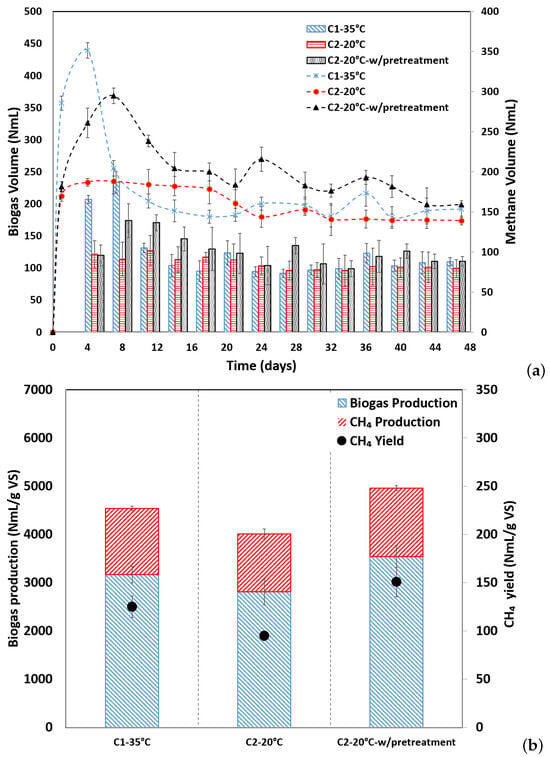
Figure 1
Open AccessArticle
Energy Security Blind Spots of Gas, Oil, and Coal Exporters
by
Andrew Curtis and Benjamin McLellan
Methane 2024, 3(1), 191-213; https://doi.org/10.3390/methane3010012 - 12 Mar 2024
Abstract
►▼
Show Figures
The global narrative around domestic energy security is dominated by the paradigm of import-dependent countries, and as a result the interactions of energy export activities with domestic energy systems are not generally considered. In this paper, we apply a systems approach to establish
[...] Read more.
The global narrative around domestic energy security is dominated by the paradigm of import-dependent countries, and as a result the interactions of energy export activities with domestic energy systems are not generally considered. In this paper, we apply a systems approach to establish two potential blind spots in evaluating the whole-of-system energy security of energy resource exporters (actual primary energy self-sufficiency and export exposure of the domestic energy system), and examine some case studies, primarily in the Australian context, to validate the existence of these blind spots. The commencement of LNG exports from the state of Queensland is examined in detail. Furthermore, we propose two novel quantitative indicators to mitigate the blind spots established. First, a revised method is proposed to calculate energy self-sufficiency, showing for the exporters studied a less secure position than shown by the traditional method. Second, an indicator is proposed to quantify the extent of exposure of the domestic energy system to international markets through export linkages, which we have applied to Australia’s domestic energy system, showing the extent of the increase in international exposure since LNG exports from Queensland commenced in 2015–2016. Conclusions of this paper include the realization that domestic energy security for energy exporters, such as Australia and the other countries examined, is more complex and, in the cases examined, less secure than importer-oriented energy security frameworks have previously recognized. A further conclusion is established that the decoupling of energy resource exports from the domestic energy system through transition to a zero-carbon energy system based on domestic renewable energy sources can be an effective means of improving Australia’s energy security.
Full article
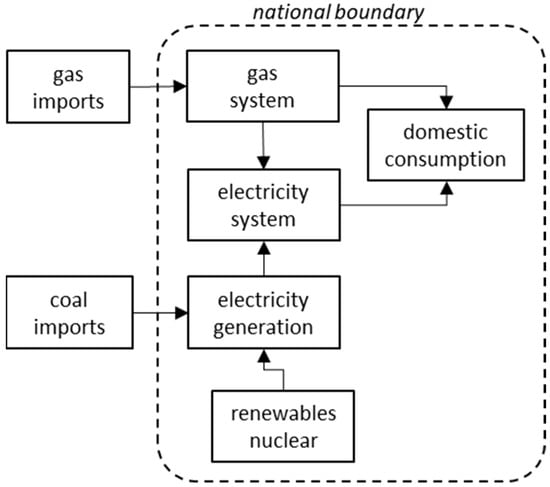
Figure 1
Open AccessReview
Exploring Geochemical Signatures in Production Water: Insights from Coal Bed Methane and Shale Gas Exploration—A Brief Review
by
Santanu Ghosh, Tushar Adsul, Balram Tiwari, Dinesh Kumar and Atul Kumar Varma
Methane 2024, 3(1), 172-190; https://doi.org/10.3390/methane3010011 - 04 Mar 2024
Abstract
►▼
Show Figures
This article furnishes a brief review of the geochemistry of waters produced during coal bed methane and shale gas exploration. Stable deuterium and oxygen isotopes of produced waters, as well as the stable carbon isotope of dissolved inorganic carbon in these waters, are
[...] Read more.
This article furnishes a brief review of the geochemistry of waters produced during coal bed methane and shale gas exploration. Stable deuterium and oxygen isotopes of produced waters, as well as the stable carbon isotope of dissolved inorganic carbon in these waters, are influenced by groundwater recharge, methanogenic pathways, the mixing of formation water with saline water, water–rock interactions, well completion, contamination from water from adjacent litho-units, and coal bed dewatering, among many others. Apart from the isotopic fingerprints, significant attention should be given to the chemistry of produced waters. These waters comprise natural saturated and aromatic organic functionalities, metals, radioisotopes, salts, inorganic ions, and synthetic chemicals introduced during hydraulic fracturing. Hence, to circumvent their adverse environmental effects, produced waters are treated with several technologies, like electro-coagulation, media filtration, the coupling of chemical precipitation and dissolved air flotation, electrochemical Fe+2/HClO oxidation, membrane distillation coupled with the walnut shell filtration, etc. Although produced water treatment incurs high costs, some of these techniques are economically feasible and sustain unconventional hydrocarbon exploitation.
Full article
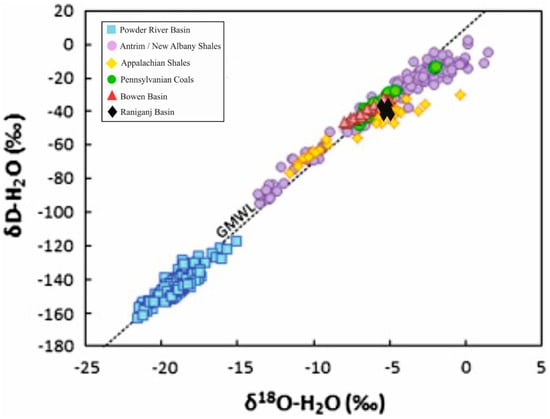
Figure 1
Open AccessFeature PaperArticle
Effect of Particle Size on the Biomethanation Kinetics of Mechanically Pretreated Sargassum spp. Biomass
by
Rosy Paletta, Rossella Girimonte, Yessica A. Castro, Jose Atilio De Frias and Vincenza Calabrò
Methane 2024, 3(1), 160-171; https://doi.org/10.3390/methane3010010 - 04 Mar 2024
Abstract
The collection and use of Sargassum spp. as feedstock for the production of valuable products such as biomethane by anaerobic digestion (AD) would mitigate the negative impact of the blooms and the costs related to waste management in the Dominican Republic. In this
[...] Read more.
The collection and use of Sargassum spp. as feedstock for the production of valuable products such as biomethane by anaerobic digestion (AD) would mitigate the negative impact of the blooms and the costs related to waste management in the Dominican Republic. In this work, the effect of the particle size of pelagic Sargassum spp. biomass, as a result of mechanical pretreatments, on the biomethanation was determined. The granulometric analysis of the mechanically pre-treated biomass was carried out using a Mastersize2000. The Biochemical Methane Potential (BMP) of the samples was determined using an Automatic Potential System Test II (AMPTS® II). The kinetic parameters of the reaction were scientifically evaluated by using First order kinetic Model and modified Gompertz Model. The granulometric analysis showed a monomodal distribution on crushed biomass (505 µm) and a bimodal distribution on the milling sample (107 µm). The bimodal biomass means the biomass is characterized by the presence of fine and large particles. We observed that BMP increased by 78.85% when particles were reduced from 50,000 µm to 505 µm and by 73.61% when particles were reduced from 50,000 µm to 107 µm. A low methane yield from the milling biomass (107 µm) compared to the crushed biomass (505 µm) could be related to the excessive reduction of particle size. The fine particles are subject to the formation of aggregates and consequently, the contact area between the algae cells and the microorganisms that operate the anaerobic digestion process decreases.
Full article
(This article belongs to the Special Issue Anaerobic Digestion Process: Converting Waste to Energy)
►▼
Show Figures

Figure 1
Open AccessArticle
Use of Increasing Levels of Low-Quality Forage in Dairy Cows’ Diets to Regulate Enteric Methane Production in Subtropical Regions
by
Mohammed Benaouda, Manuel González-Ronquillo, Francisca Avilés-Nova, Reynaldo Zaragoza-Guerrero, Juan Carlos Ku-Vera and Octavio Alonso Castelán-Ortega
Methane 2024, 3(1), 149-159; https://doi.org/10.3390/methane3010009 - 22 Feb 2024
Abstract
Dairy cows are the highest daily and annual methane (CH4) producers among all cattle categories. So, the present study aimed to evaluate the effect of increasing supplementation levels of a low-quality forage on dry matter intake (DMI), DM digestibility (DMD), milk
[...] Read more.
Dairy cows are the highest daily and annual methane (CH4) producers among all cattle categories. So, the present study aimed to evaluate the effect of increasing supplementation levels of a low-quality forage on dry matter intake (DMI), DM digestibility (DMD), milk production, enteric CH4 emission, gross energy, and protein partitioning in Holstein cows. In total, eight cows (112 ± 38 days postpartum; mean ± s.d.) were randomly assigned to 4 treatments composed of 4 dietary neutral detergent fibre (NDF) inclusion levels (40.2% (control), 43.3%, 46.5%, and 50.5%) in a 4 × 4 repeated Latin square experimental design. The cows were fed corn + alfalfa silage and a concentrate (60:40 forage:concentrate ratio). To increase the contents of low-quality NDF, part of the silage was replaced with maize stover (MSTV). The CH4 production was measured in an open-circuit respiration chamber. The DMI increased significantly and linearly (p < 0.05) with increasing levels of MSTV. However, the CH4 yield decreased (p < 0.0001) as the NDF level increased (32.1, 28.1, 23.1, and 21.3 CH4 L/kg DMI, respectively). DMD decreased as NDF levels in the diet increased (p < 0.0001). The NDF digestibility (DNDF) explained the better (p < 0.0001) CH4 production response than DMD. It was concluded that low-quality forages can be used to regulate CH4 production in subtropical and tropical climate regions.
Full article
Open AccessFeature PaperReview
Methane Biofiltration Processes: A Summary of Biotic and Abiotic Factors
by
Fatemeh Ahmadi, Tatiana Bodraya and Maximilian Lackner
Methane 2024, 3(1), 122-148; https://doi.org/10.3390/methane3010008 - 21 Feb 2024
Abstract
The ongoing yearly rise in worldwide methane (CH4) emissions is mostly due to human activities. Nevertheless, since over half of these emissions are scattered and have a concentration of less than 3% (v/v), traditional physical–chemical methods are
[...] Read more.
The ongoing yearly rise in worldwide methane (CH4) emissions is mostly due to human activities. Nevertheless, since over half of these emissions are scattered and have a concentration of less than 3% (v/v), traditional physical–chemical methods are not very effective in reducing them. In this context, biotechnologies like biofiltration using methane-consuming bacteria, also known as methanotrophs, offer a cost-efficient and practical approach to addressing diffuse CH4 emissions. The present review describes recent findings in biofiltration processes as one of the earliest biotechnologies for treating polluted air. Specifically, impacts of biotic (such as cooperation between methanotrophs and non-methanotrophic bacteria and fungi) and abiotic factors (such as temperature, salinity, and moisture) that influence CH4 biofiltration were compiled. Understanding the processes of methanogenesis and methanotrophy holds significant importance in the development of innovative agricultural practices and industrial procedures that contribute to a more favourable equilibrium of greenhouse gases. The integration of advanced genetic analyses can enable holistic approaches for unravelling the potential of biological systems for methane mitigation. This study pioneers a holistic approach to unravelling the biopotential of methanotrophs, offering unprecedented avenues for biotechnological applications.
Full article
(This article belongs to the Special Issue Trends in Methane-Based Biotechnology)
►▼
Show Figures

Figure 1
Open AccessArticle
Genetical and Biochemical Basis of Methane Monooxygenases of Methylosinus trichosporium OB3b in Response to Copper
by
Dipayan Samanta, Tanvi Govil, Priya Saxena, Lee Krumholz, Venkataramana Gadhamshetty, Kian Mau Goh and Rajesh K. Sani
Methane 2024, 3(1), 103-121; https://doi.org/10.3390/methane3010007 - 20 Feb 2024
Abstract
Over the past decade, copper (Cu) has been recognized as a crucial metal in the differential expression of soluble (sMMO) and particulate (pMMO) forms of methane monooxygenase (MMO) through a mechanism referred to as the “Cu switch”. In this study, we used Methylosinus
[...] Read more.
Over the past decade, copper (Cu) has been recognized as a crucial metal in the differential expression of soluble (sMMO) and particulate (pMMO) forms of methane monooxygenase (MMO) through a mechanism referred to as the “Cu switch”. In this study, we used Methylosinus trichosporium OB3b as a model bacterium to investigate the range of Cu concentrations that trigger the expression of sMMO to pMMO and its effect on growth and methane oxidation. The Cu switch was found to be regulated within Cu concentrations from 3 to 5 µM, with a strict increase in the methane consumption rates from 3.09 to 3.85 µM occurring on the 6th day. Our findings indicate that there was a decrease in the fold changes in the expression of methanobactin (Mbn) synthesis gene (mbnA) with a higher Cu concentration, whereas the Ton-B siderophore receptor gene (mbnT) showed upregulation at all Cu concentrations. Furthermore, the upregulation of the di-heme enzyme at concentrations above 5 µM Cu may play a crucial role in the copper switch by increasing oxygen consumption; however, the role has yet not been elucidated. We developed a quantitative assay based on the naphthalene–Molisch principle to distinguish between the sMMO- and pMMO-expressing cells, which coincided with the regulation profile of the sMMO and pMMO genes. At 0 and 3 µM Cu, the naphthol concentration was higher (8.1 and 4.2 µM, respectively) and gradually decreased to 0 µM naphthol when pMMO was expressed and acted as the sole methane oxidizer at concentrations above 5 µM Cu. Using physical protein–protein interaction, we identified seven transporters, three cell wall biosynthesis or degradation proteins, Cu resistance operon proteins, and 18 hypothetical proteins that may be involved in Cu toxicity and homeostasis. These findings shed light on the key regulatory genes of the Cu switch that will have potential implications for bioremediation and biotechnology applications.
Full article
(This article belongs to the Special Issue Trends in Methane-Based Biotechnology)
►▼
Show Figures
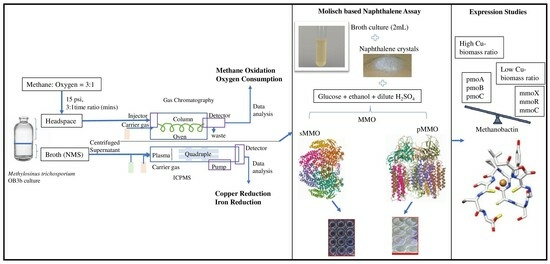
Graphical abstract
Open AccessReview
Research Progress on Stability Control on Ni-Based Catalysts for Methane Dry Reforming
by
Minghui Wei and Xuerong Shi
Methane 2024, 3(1), 86-102; https://doi.org/10.3390/methane3010006 - 06 Feb 2024
Abstract
CO2 reforming of CH4 (DRM) utilizes the greenhouse gases of CH4 and CO2 to obtain the synthesis gas, benefiting the achievement of carbon neutrality. However, the deactivation of Ni-based catalysts caused by sintering and carbon deposition limits the industrial
[...] Read more.
CO2 reforming of CH4 (DRM) utilizes the greenhouse gases of CH4 and CO2 to obtain the synthesis gas, benefiting the achievement of carbon neutrality. However, the deactivation of Ni-based catalysts caused by sintering and carbon deposition limits the industrial application. Focusing on stability improvement, this review first summarizes the reaction mechanism and deactivation mechanism in DRM and then discusses the impact of catalyst active components, supports, and interfacial structure. Finally, we propose the design direction of stable Ni-based catalysts towards DRM, providing guidance for the future development of catalysts suitable for industrial production.
Full article
(This article belongs to the Special Issue Methane Dry Reforming)
►▼
Show Figures
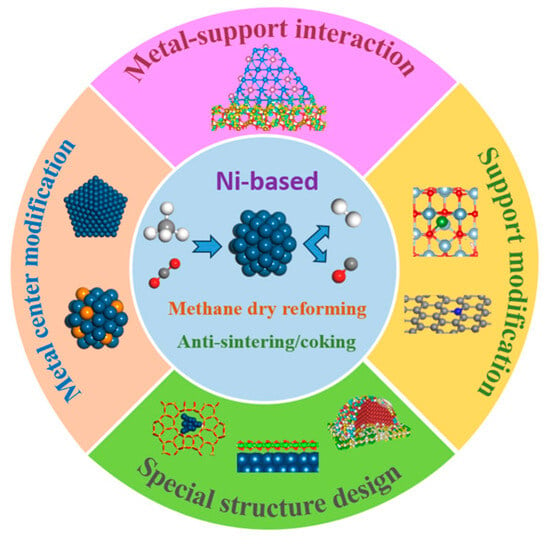
Figure 1
Open AccessArticle
Towards a Mechanistic Understanding of the Slagging Propensities of Petroleum Coke: Lessons Learned from Its Co-Combustion with Natural Gas in Oxygen-Enriched Atmospheres
by
Nghia Duc Tin Nguyen and Gautham Krishnamoorthy
Methane 2024, 3(1), 65-85; https://doi.org/10.3390/methane3010005 - 24 Jan 2024
Abstract
►▼
Show Figures
A Computational Fluid Dynamic study was carried out to match the measured outer ash deposition rates associated with the combustion of petroleum coke (PC)–natural gas in AIR and O2/CO2 (70/30 vol%, OXY70). The fly ash PSD associated with high-fixed-carbon, non-porous
[...] Read more.
A Computational Fluid Dynamic study was carried out to match the measured outer ash deposition rates associated with the combustion of petroleum coke (PC)–natural gas in AIR and O2/CO2 (70/30 vol%, OXY70). The fly ash PSD associated with high-fixed-carbon, non-porous fuel was estimated using a shrinking sphere burnout model and employed in conjunction with particle kinetic energy (PKE), particle viscosity (µP), and a critical Weber-number-based capture criterion. Deposition rate predictions were sensitive to the fly ash composition employed for estimating µP due to the significant enrichment of Fe in the deposits. Predictions were insensitive to the specific µP model formulation employed or whether the V2O5 in the ash was assumed to play the role of a glass former or a glass modifier. OXY70 scenario impaction rates were significantly lower than the measured deposition rates when the fly ash PSD associated with the AIR scenario was employed in the calculations. This necessitated an ad hoc modification of the OXY70 fly ash PSD to a coarser range to match the measurements and attributing it to agglomeration resulting from longer residence times and higher temperatures. This shift in PSD was in line with AIR and OXY70 fly ash PSD measurements reported previously.
Full article
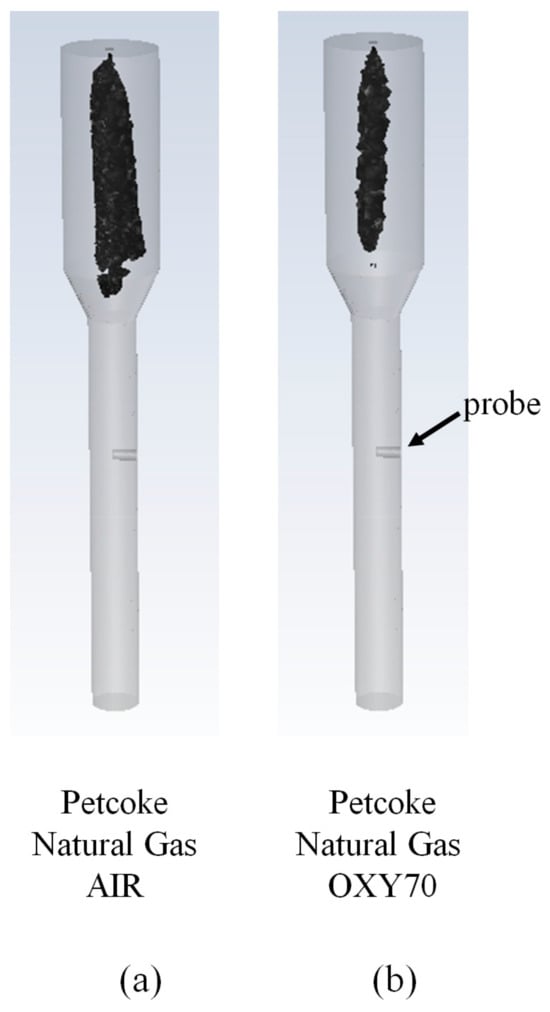
Figure 1
Open AccessArticle
High-Pressure Hydrogenation: A Path to Efficient Methane Production from CO2
by
Maitê L. Gothe, Adolfo L. Figueredo, Laís R. Borges, Ruben Ramos, Andreia F. Peixoto and Pedro Vidinha
Methane 2024, 3(1), 53-64; https://doi.org/10.3390/methane3010004 - 15 Jan 2024
Abstract
►▼
Show Figures
Methane has a rather relevant role in the “Power-to-Gas” concept, which is central in the current paradigm of climate change and renewable energies. Methane, the main component of natural gas, can be produced by catalytic hydrogenation reactions, particularly of CO2. A
[...] Read more.
Methane has a rather relevant role in the “Power-to-Gas” concept, which is central in the current paradigm of climate change and renewable energies. Methane, the main component of natural gas, can be produced by catalytic hydrogenation reactions, particularly of CO2. A very effective catalyst in this reaction, hydrotalcite-derived nickel nanoparticles supported on alumina, Ni/Al2O3-HTC, can be employed in a high-pressure flow reactor to convert CO2 and H2 into CH4 at 100% selectivity and 84% conversion, whereas at atmospheric pressure, methane can be obtained with up to 90% selectivity. The high-pressure aspect also allows fast-paced production—over 5 m3·h−1·kgcat−1 of CH4 can be generated.
Full article
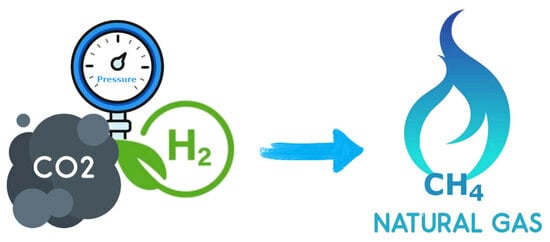
Graphical abstract
Open AccessReview
A Review on Dry Anaerobic Digestion: Existing Technologies, Performance Factors, Challenges, and Recommendations
by
Umer Hayyat, Muhammad Usman Khan, Muhammad Sultan, Umair Zahid, Showkat Ahmad Bhat and Mohd Muzamil
Methane 2024, 3(1), 33-52; https://doi.org/10.3390/methane3010003 - 15 Jan 2024
Abstract
With the increase in the growing rate of municipal solid waste throughout the world and due to the high moisture and organic components of the organic fraction of municipal solid waste, dry anaerobic digestion has become the future direction to cope with this
[...] Read more.
With the increase in the growing rate of municipal solid waste throughout the world and due to the high moisture and organic components of the organic fraction of municipal solid waste, dry anaerobic digestion has become the future direction to cope with this waste while reducing the impact on the environment, including climate change. Dry anaerobic digestion has become a promising technology that converts the organic fraction of municipal solid waste into combustible biogases, which can be used as an alternative energy source. However, the technology faces several challenges that must be addressed to enhance its performance and adoption. This paper provides a comprehensive analysis of the current technologies used for dry anaerobic digestion in OFMSW and delves into the various factors that influence the performance of these technologies. This review paper also identifies and discusses the challenges faced in optimizing and scaling up these technologies, such as feedstock pretreatment requirements, characteristics of inoculum, and other crucial parameters.
Full article
(This article belongs to the Special Issue Anaerobic Digestion Process: Converting Waste to Energy)
►▼
Show Figures
Figure 1
Open AccessFeature PaperArticle
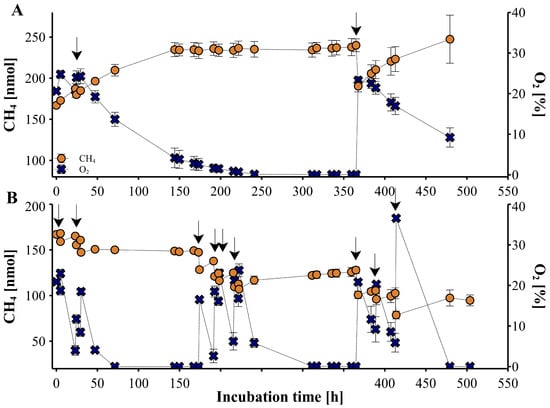
The Trade-Off between Enteric and Manure Methane Emissions and Their Bacterial Ecology in Lactating Cows Fed Diets Varying in Forage-to-Concentrate Ratio and Rapeseed Oil
by
Babak Darabighane, Ilma Tapio, Saija Rasi, Ari-Matti Seppänen, Lucia Blasco, Seppo Ahvenjärvi and Ali R. Bayat
Methane 2024, 3(1), 12-32; https://doi.org/10.3390/methane3010002 - 09 Jan 2024
Cited by 1
Abstract
An experiment was conducted to examine how dietary interventions reducing enteric methane (CH4) emissions influence manure CH4 emissions in biogas production (as biochemical methane potential (BMP)) or under static conditions mimicking natural manure storage conditions. Experimental treatments consisted of a
[...] Read more.
An experiment was conducted to examine how dietary interventions reducing enteric methane (CH4) emissions influence manure CH4 emissions in biogas production (as biochemical methane potential (BMP)) or under static conditions mimicking natural manure storage conditions. Experimental treatments consisted of a factorial arrangement of high (HF: 0.65) or low (LF: 0.35) levels of forage and 0 or 50 g of rapeseed oil per kg of diet dry matter. Oil supplementation reduced daily enteric CH4 emissions, especially in the HF diet, by 20%. Greater dietary concentrate proportion reduced CH4 yield and intensity (6 and 12%, respectively) and decreased pH, increased total volatile fatty acids, and molar proportions of butyrate and valerate in feces incubated under static conditions. Oil supplementation increased daily BMP and BMP calculated per unit of organic matter (OM) (17 and 15%, respectively). Increased dietary concentrate had no impact on daily BMP and BMP per unit of OM, whereas it reduced daily CH4 production by 89% and CH4 per unit of OM by 91% under static conditions. Dietary oil supplementation tended to decrease fecal CH4 production per unit of digestible OM (23%) under static conditions. Diets had no impact on the alpha diversity of ruminal prokaryotes. After incubation, the fecal prokaryote community was significantly less diverse. Diets had no effect on alpha diversity in the BMP experiment, but static trial fecal samples originating from the HF diet showed significantly lower diversity compared with the LF diet. Overall, the tested dietary interventions reduced enteric CH4 emissions and reduced or tended to reduce manure CH4 emissions under static conditions, indicating a lack of trade-off between enteric and manure CH4 emissions. The potential for increasing CH4 yields in biogas industries due to dietary interventions could lead to a sustainable synergy between farms and industry.
Full article
(This article belongs to the Special Issue Anaerobic Digestion Process: Converting Waste to Energy)
►▼
Show Figures
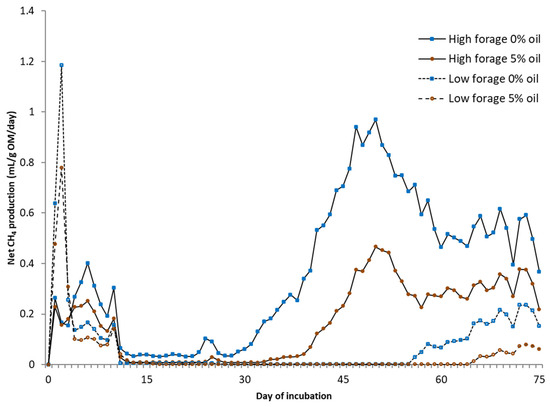
Figure 1
Open AccessReview
Relationships between Dietary Chemical Components and Enteric Methane Production and Application to Diet Formulation in Beef Cattle
by
Michael L. Galyean and Kristin E. Hales
Methane 2024, 3(1), 1-11; https://doi.org/10.3390/methane3010001 - 09 Jan 2024
Abstract
►▼
Show Figures
We used published data consisting of 263 treatment mean observations from beef cattle and dairy steers and heifers, in which CH4 was measured via chambers or head boxes, to evaluate relationships between enteric CH4 production and dry matter intake (DMI) and
[...] Read more.
We used published data consisting of 263 treatment mean observations from beef cattle and dairy steers and heifers, in which CH4 was measured via chambers or head boxes, to evaluate relationships between enteric CH4 production and dry matter intake (DMI) and dietary components. Daily DMI was positively related (slope = 15.371, p < 0.001) to total daily production (g/d) of CH4 (r2 = 0.821). Among chemical components, dietary neutral detergent fiber (NDF) concentration was the most highly related (r2 = 0.696; slope = 0.2001; p < 0.001) to CH4 yield (g/kg of DMI), with strong relationships also noted for dietary starch:NDF ratio (r2 = 0.662; slope = −2.4587; p < 0.001), starch (r2 = 0.495; slope = −0.106; p < 0.001), and the proportion of metabolizable energy relative to gross energy (r2 = 0.561; slope = −23.663; p < 0.001). The slope (−0.5871) and intercept (22.2295) for the dietary ether extract vs. CH4 yield were significant (p < 0.001), but the relationship was highly variable (r2 = 0.150). For dietary crude protein concentration, the slope for CH4 yield was not significant (−0.0344; p < 0.381) with an r2 value near zero. Decreasing DMI by programming body weight gain or restricting feed intake could decrease CH4 production in confined cattle, but these approaches might negatively affect growth performance and product quality, potentially negating positive effects on CH4 production. Feeding higher-quality forages or using grazing management systems that decrease dietary NDF concentrations or substituting grain (starch) for forage should decrease both CH4 yield from enteric production and manure CH4 production via increased digestibility. Effects of feeding management and diet formulation strategies should be additive with other mitigation approaches such as feed additives, allowing the cattle industry to achieve maximal decreases in enteric CH4 production, while concurrently maintaining optimal beef production.
Full article
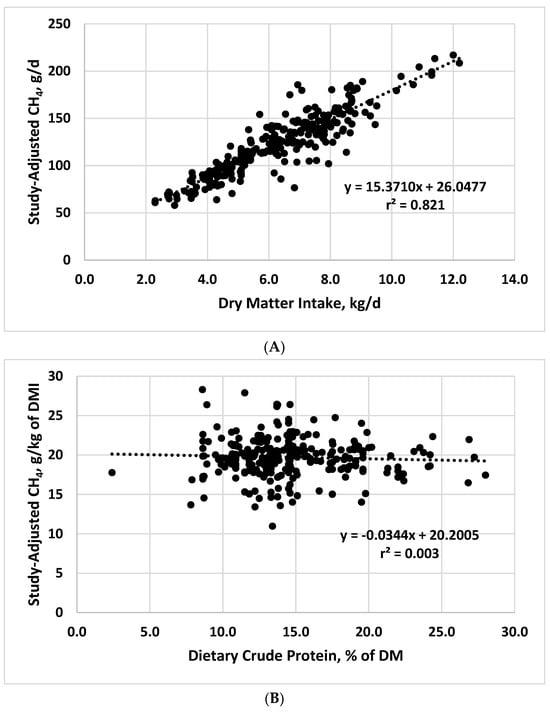
Figure 1
Open AccessArticle
Dry Reforming of Methane over Li-Doped Ni/TiO2 Catalysts: Effect of Support Basicity
by
Vicente Pérez-Madrigal, Edna Ríos-Valdovinos, Elizabeth Rojas-García, Miguel A. Valenzuela and Francisco Pola-Albores
Methane 2023, 2(4), 452-469; https://doi.org/10.3390/methane2040031 - 15 Dec 2023
Abstract
In this research, we investigate the impact of Li doping on a TiO2 support, synthesized through the sol-gel method, with a focus on varying the aging time. Our objective is to elucidate how aging duration and doping influence the surface basicity, thereby
[...] Read more.
In this research, we investigate the impact of Li doping on a TiO2 support, synthesized through the sol-gel method, with a focus on varying the aging time. Our objective is to elucidate how aging duration and doping influence the surface basicity, thereby mitigating carbon formation and amplifying the catalytic efficacy of Ni-loaded catalysts (15 wt.%). Essential characterization techniques encompass X-ray diffraction, H2-TPR, FE-SEM, N2-physisorption, DLS, FTIR, and Raman spectroscopies. Our findings reveal that extended aging periods promote the development of a basic character, attributable to oxygen defects within TiO2. This inherent trait bears significant implications for catalyst performance, stability, and carbon formation during the reaction. Remarkably, the catalyst with the highest catalytic activity and stability boasts an 85% relative basicity, a property also induced by incorporating lithium into the TiO2 support.
Full article
(This article belongs to the Special Issue Methane Dry Reforming)
►▼
Show Figures
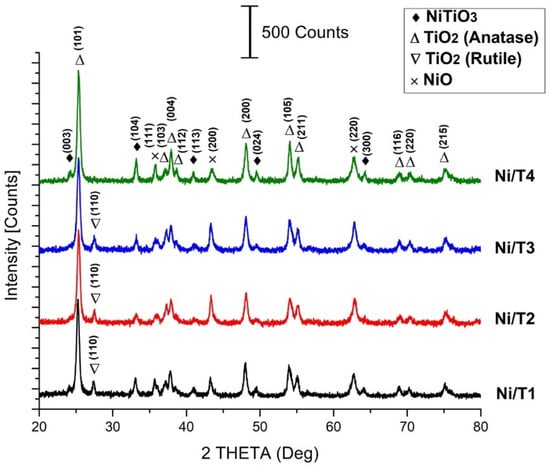
Figure 1
Open AccessFeature PaperArticle
Using Ground- and Drone-Based Surface Emission Monitoring (SEM) Data to Locate and Infer Landfill Methane Emissions
by
Tarek Abichou, Nizar Bel Hadj Ali, Sakina Amankwah, Roger Green and Eric S. Howarth
Methane 2023, 2(4), 440-451; https://doi.org/10.3390/methane2040030 - 11 Dec 2023
Abstract
►▼
Show Figures
Ground- and drone-based surface emission monitoring (SEM) campaigns were performed at two municipal solid waste landfills, during the same week as mobile tracer correlation method (TCM) testing was used to measure the total methane emissions from the same landfills. The G-SEM and the
[...] Read more.
Ground- and drone-based surface emission monitoring (SEM) campaigns were performed at two municipal solid waste landfills, during the same week as mobile tracer correlation method (TCM) testing was used to measure the total methane emissions from the same landfills. The G-SEM and the D-SEM data, along with wind data, were used as input into an inverse modeling approach combined with an optimization-based methane emission estimation method (implemented in a tool called SEM2Flux). This approach involves the use of backward dispersion modeling to estimate the whole-site methane emissions from a given landfill and the identification of locations and emission rates of major leaks. SEM2Flux is designed to exploit the measured surface methane concentration concurrently with wind data and tackle two problems: (1) inferring the estimates of methane rates from individual landfills, and (2) identifying the likely locations of the main emission sources. SEM2Flux results were also compared with emission estimates obtained using TCM. In Landfill B, the average TCM-measured methane emissions was 1178 Kg/h, with a standard deviation of 271 Kg/h. In Landfill C, the average TCM-measured emission rate was 601 Kg/h, with a standard deviation of 292 Kg/h. For both landfills, the D-SEM data yielded statistically similar estimates of methane emissions as the TCM-measured emissions. On the other hand, the G-SEM data yielded comparable estimates of emissions to TCM-measured emissions only for Landfill C, where the D-SEM and G-SEM data were statistically not different. The results of this study showcase the ability of this method using surface concentrations to provide a rapid and simple estimation of fugitive methane emissions from landfills. Such an approach can also be used to assess the effectiveness of different remedial actions in reducing fugitive methane emissions from a given landfill.
Full article

Figure 1
Open AccessArticle
Anaerobic Co-Digestion of Vinasse and Pentose Liquor and the Role of Micronutrients in Methane Production within Sugarcane Biorefineries
by
Gabriela P. Freitas, Brenno Vinicius M. Lima, Maria Paula C. Volpi, Renata P. Rodriguez and Bruna S. Moraes
Methane 2023, 2(4), 426-439; https://doi.org/10.3390/methane2040029 - 08 Dec 2023
Abstract
►▼
Show Figures
Anaerobic digestion (AD) of residues from integrated first- and second-generation ethanol (1G2G) biorefineries is a sustainable method for energy recovery through biogas production. This study evaluated the co-digestion of 1G vinasse, 2G vinasse and pentose liquor (from the pretreatment of sugarcane bagasse for
[...] Read more.
Anaerobic digestion (AD) of residues from integrated first- and second-generation ethanol (1G2G) biorefineries is a sustainable method for energy recovery through biogas production. This study evaluated the co-digestion of 1G vinasse, 2G vinasse and pentose liquor (from the pretreatment of sugarcane bagasse for 2G ethanol production) compared to individual digestions using biochemical methane potential (BMP) assays. The results showed some “key” micronutrients from the substrates that affected methane (CH4) production, while their balance provided by co-digestion achieved high digestibility (95%). High iron (Fe) and nickel (Ni) concentrations, in addition to furfural (0.33 g L−1) in pentose liquor seemed to decrease its CH4 production potential. Despite these adverse effects observed in mono-digestion, co-digestion was beneficial for this substrate, increasing digestibility (52%) and BMP (118%). The highest BMP was observed in vinasse 2G (631 ± 6 NmL CH4 gTVS−1), with no significant difference compared to the adjusted modified Gompertz model (624 ± 10 NmL CH4 gTVS−1). The co-digestion system also presented the highest specific CH4 production rate (20 ± 1 NmL CH4 gTVS−1day−1) and shortened the lag phase by 19% compared to the AD of isolated 1G vinasse with the second lowest BMP value (494 ± 11 NmL CH4 gTVS−1).
Full article
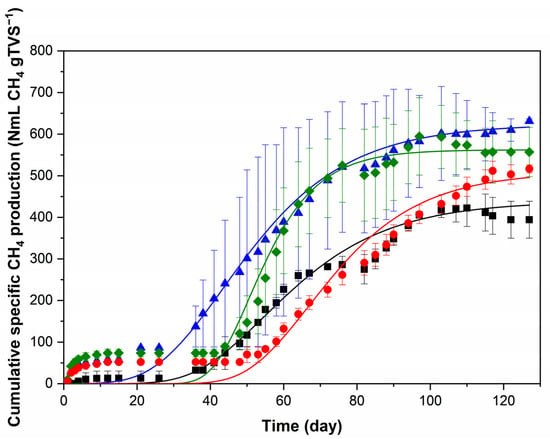
Figure 1
Open AccessFeature PaperArticle
Density Functional Theory Insight into Chemical Vapor Infiltration
by
Eric A. Walker, Joseph J. Marziale and James Chen
Methane 2023, 2(4), 415-425; https://doi.org/10.3390/methane2040028 - 09 Nov 2023
Cited by 1
Abstract
►▼
Show Figures
Chemical Vapor Infiltration (CVI) has proven remarkably successful in producing strong and lightweight ceramic matrix composite materials. This technology has matured to regular industrial use. However, two fundamental problems remain, and those are the formation of pores and depositing of weaker material than
[...] Read more.
Chemical Vapor Infiltration (CVI) has proven remarkably successful in producing strong and lightweight ceramic matrix composite materials. This technology has matured to regular industrial use. However, two fundamental problems remain, and those are the formation of pores and depositing of weaker material than silicon carbide (
Figure 1
Open AccessFeature PaperReview
Methane Removal from Air: Challenges and Opportunities
by
Jin Wang and Qinghua Peter He
Methane 2023, 2(4), 404-414; https://doi.org/10.3390/methane2040027 - 01 Nov 2023
Abstract
►▼
Show Figures
Driven by increasing greenhouse gas (GHG) concentrations in the atmosphere, extreme weather events have become more frequent and their impacts on human lives have become more severe. Therefore, the need for short-term GHG mitigations is urgent. Recently, methane has been recognized as an
[...] Read more.
Driven by increasing greenhouse gas (GHG) concentrations in the atmosphere, extreme weather events have become more frequent and their impacts on human lives have become more severe. Therefore, the need for short-term GHG mitigations is urgent. Recently, methane has been recognized as an important mitigation target due to its high global warming potential (GWP). However, methane’s low concentration in the atmosphere and stable molecular structure make its removal from the air highly challenging. This review first discusses the fundamental aspects of the challenges in atmospheric methane removal and then briefly reviews the existing research strategies following the mechanisms of natural methane sinks. Although still in its infancy, recent research on methane removal from the air holds great potential for slowing down global warming. At the same time, it is important to carefully examine the energy consumption of these methane removal strategies and whether they will be able to achieve net GHG reduction. In addition, due to the scale of methane removal from the air, any potential solution’s environmental impacts must be carefully evaluated before it can be implemented in practice.
Full article
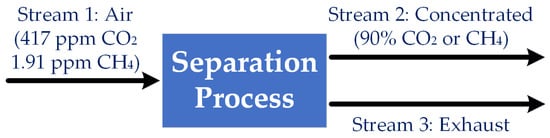
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
Methane
Trends in Methane-Based Biotechnology
Guest Editors: Yadira Rodríguez, Juan Carlos López, Maximilian LacknerDeadline: 30 April 2024
Special Issue in
Methane
Perspective in Natural Gas Hydrate
Guest Editors: Jing-Chun Feng, Yi WangDeadline: 30 June 2024
Special Issue in
Methane
Methane Dry Reforming
Guest Editors: Oz M. Gazit, Brian A. RosenDeadline: 31 August 2024