Practice and Research in Clinical Pharmacology
A topical collection in Journal of Clinical Medicine (ISSN 2077-0383). This collection belongs to the section "Pharmacology".
Viewed by 71175Editors
Interests: drug individualization; clinical trials; clinical pharmacology
Topical Collection Information
Dear Colleagues,
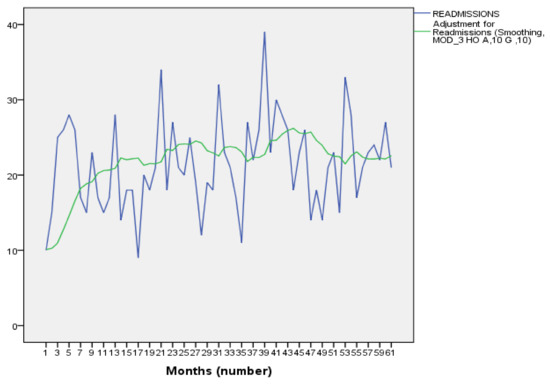
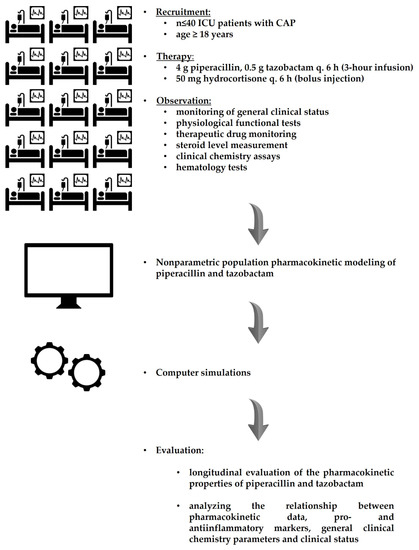
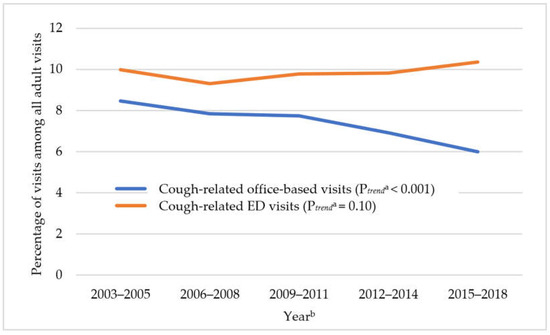
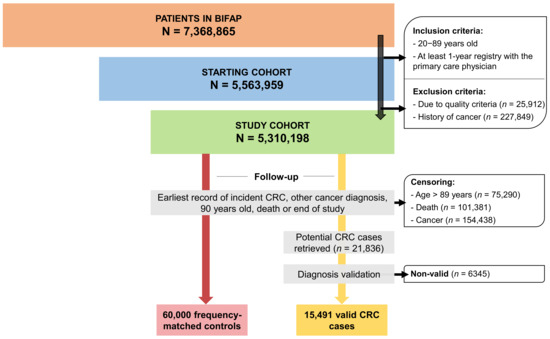
Clinical pharmacology is a medical discipline whose main interest is to assess the benefit–risk balance of drug therapy at both population and individual levels in order to provide the best possible therapy to patients and to the right dose. Thus, it involves on one hand the scientific evaluation of drugs during clinical development (clinical trials and drug regulation) and clinical use (drug utilization, comparative effectiveness, pharmacovigilance, pharmacoeconomics and drug policies). On the other hand, it is a medical discipline devoted to direct patient care through drug individualization (therapeutic drug monitoring, pharmacogenetics and system clinical pharmacology) and consultancy on drug-related problems (adverse drug reactions, interactions, drug toxicology, drug selection and individual benefit–risk assessment).
Journal of Clinical Medicine has given us the opportunity to show the scope of clinical pharmacology to a broad audience and the relevance of this discipline in clinical practice. Therefore, we are looking for outstanding original manuscripts and reviews showing the broad and transversal character of clinical pharmacology as a scientific and medical discipline, especially for manuscripts dealing with all those aspects of clinical pharmacology with a relevant impact on patient care.
Dr. Antonio J. Carcas-Sansuán
Dr. Alberto M. Borobia Pérez
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Clinical Medicine is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Clinical Pharmacology
- Clinical Trials
- Drug Development
- Drug Safety
- Pharmacovigilance
- Toxicology
- Therapeutic Drug Monitoring
- Pharmacogenetics
- Drug Individualization
- Drug Policy
- Benefit-Risk Assessment