Journal Description
Immuno
Immuno
is an international, peer-reviewed, open access journal on immunological research and clinical applications published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, EBSCO, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.7 days after submission; acceptance to publication is undertaken in 4.4 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
Anti-Inflammatory Efficacy of Resveratrol-Enriched Rice Callus Extract on Lipopolysaccharide-Stimulated RAW264.7 Macrophages
Immuno 2024, 4(2), 131-146; https://doi.org/10.3390/immuno4020009 - 03 Apr 2024
Abstract
Resveratrol and its derivative piceid exhibit a wide spectrum of health-promoting bioactivities. A resveratrol-enriched variety of Dongjin rice (DJ526) has been developed by transfection of a resveratrol biosynthesis gene, and increased resveratrol content has been confirmed in seeds following germination. In the current
[...] Read more.
Resveratrol and its derivative piceid exhibit a wide spectrum of health-promoting bioactivities. A resveratrol-enriched variety of Dongjin rice (DJ526) has been developed by transfection of a resveratrol biosynthesis gene, and increased resveratrol content has been confirmed in seeds following germination. In the current study, these resveratrol-enriched seeds were induced to produce callus, and callus extracts were evaluated for in vitro anti-inflammatory activity. Callus cultures contained greater amounts of resveratrol and piceid than DJ526 seeds, and treatment with DJ526 callus extract significantly reduced the lipopolysaccharide (LPS)-induced production of proinflammatory mediators nitric oxide and prostaglandin E2 by RAW264.7 macrophages. The inflammation-related nuclear factor kappa B and mitogen-activated protein kinase pathways were also inhibited in DJ526 callus extract-treated RAW264.7 cells, resulting in downregulation of proinflammatory factor genes COX-2, iNOS, IL-1β, IL-6, and TNF-α. Expression of the LPS-binding toll-like receptor-4 was also markedly reduced in DJ526 callus extract-treated cells compared to DJ callus extract-treated cells. These findings demonstrate increased resveratrol and piceid content by callus culture of DJ526 rice seeds and the potent anti-inflammatory activity of resveratrol-enriched callus extract.
Full article
(This article belongs to the Special Issue Anti-inflammatory and Immunomodulatory Medicinal Plants and Their Derived Immunomodulators)
►
Show Figures
Open AccessCase Report
Fulminant Recurrent Thrombosis in a Patient with Catastrophic Antiphospholipid Syndrome and Its Thirty-Day Outcome
by
Pierpaolo Di Micco, Maurizio Dorato, Maurizio Latte, Maria D’Antò, Vittorio Luiso and Gerolamo Sibilio
Immuno 2024, 4(1), 125-130; https://doi.org/10.3390/immuno4010008 - 04 Mar 2024
Abstract
Catastrophic antiphospholipid syndrome (CAPS) is a rare clinical form of antiphospholipid syndrome (APS) associated with life-threatening complications due to simultaneous thrombosis that may affect small and large vessels. It may be localized to the venous and/or arteries at the same time, and there
[...] Read more.
Catastrophic antiphospholipid syndrome (CAPS) is a rare clinical form of antiphospholipid syndrome (APS) associated with life-threatening complications due to simultaneous thrombosis that may affect small and large vessels. It may be localized to the venous and/or arteries at the same time, and there are not available guidelines based on randomized clinical trials or large series. We here report a clinical case of CAPS with onset after resolution of oligo-symptomatic infection SARS-CoV-2, that had transient improvement with warfarin after recurrent thromboses occurred despite treatment off-label with low doses of low molecular weight heparin. Furthermore, we tried to trace a line by which a multidisciplinary team may set specific timing to have follow-up because of the high morbidity, mortality, and prolonged time of hospitalization.
Full article
Open AccessArticle
A Serological Multiplexed Immunoassay (MIA) Detects Antibody Reactivity to SARS-CoV-2 and Other Viral Pathogens in Liberia and Is Configurable as a Multiplexed Inhibition Test (MINT)
by
Brien K. Haun, Albert To, Caitlin A. Williams, Aquena Ball, Karalyn Fong, Teri Ann S. Wong, Bode Shobayo, Julius Teahton, Lauren Ching, Varney Kamara, Davidetta M. Tekah, Peter Humphrey, John Berestecky, Vivek R. Nerurkar and Axel T. Lehrer
Immuno 2024, 4(1), 108-124; https://doi.org/10.3390/immuno4010007 - 03 Mar 2024
Abstract
►▼
Show Figures
The SARS-CoV-2 pandemic ignited global efforts to rapidly develop testing, therapeutics, and vaccines. However, the rewards of these efforts were slow to reach many low- to middle-income countries (LMIC) across the African continent and globally. Therefore, two bead-based multiplexed serological assays were developed
[...] Read more.
The SARS-CoV-2 pandemic ignited global efforts to rapidly develop testing, therapeutics, and vaccines. However, the rewards of these efforts were slow to reach many low- to middle-income countries (LMIC) across the African continent and globally. Therefore, two bead-based multiplexed serological assays were developed to determine SARS-CoV-2 exposure across four counties in Liberia. This study was conducted during the summer of 2021 on 189 samples collected throughout Grand Bassa, Bong, Margibi, and Montserrado counties. Our multiplexed immunoassay (MIA) detected elevated exposure to SARS-CoV-2 and multiple variant antigens. Additionally, we detected evidence of exposure to Dengue virus serotype 2, Chikungunya virus, and the seasonal coronavirus NL63. Our multiplexed inhibition test (MINT) was developed from the MIA to observe antibody-mediated inhibition of SARS-CoV-2 spike protein binding to its cognate cellular receptor ACE-2. We detected inhibitory antibodies in the tested Liberian samples, which were collectively consistent with a convalescent serological profile. These complementary assays serve to supplement existing serological testing needs and may enhance the technical capacity of scientifically underrepresented regions globally.
Full article
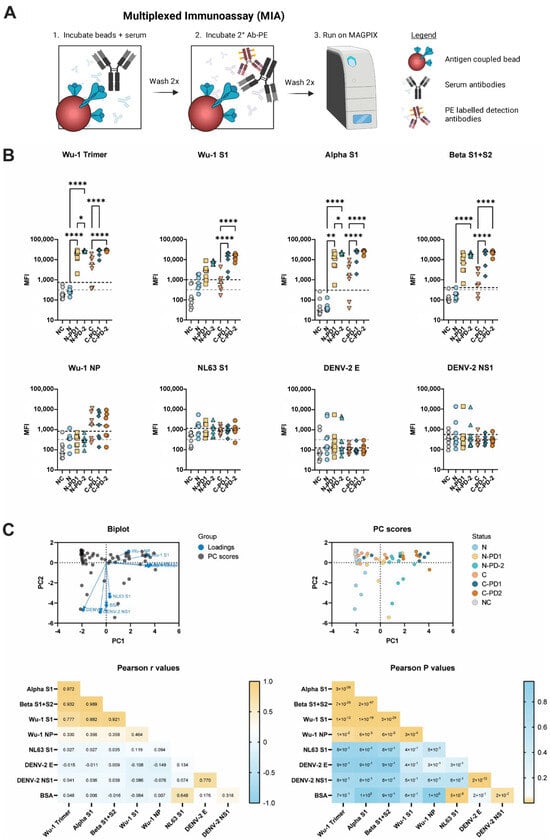
Figure 1
Open AccessReview
Exploring the Interplay between Fatty Acids, Inflammation, and Type 2 Diabetes
by
Dequina A. Nicholas, Jacques C. Mbongue, Darysbel Garcia-Pérez, Dane Sorensen, Heather Ferguson Bennit, Marino De Leon and William H. R. Langridge
Immuno 2024, 4(1), 91-107; https://doi.org/10.3390/immuno4010006 - 01 Mar 2024
Abstract
Around 285 million people worldwide currently have type 2 diabetes and it is projected that this number will be surpassed by 2030. Therefore, it is of the utmost importance to enhance our comprehension of the disease’s development. The regulation of diet, obesity, and
[...] Read more.
Around 285 million people worldwide currently have type 2 diabetes and it is projected that this number will be surpassed by 2030. Therefore, it is of the utmost importance to enhance our comprehension of the disease’s development. The regulation of diet, obesity, and inflammation in type 2 diabetes is believed to play a crucial role in enhancing insulin sensitivity and reducing the risk of onset diabetes. Obesity leads to an increase in visceral adipose tissue, which is a prominent site of inflammation in type 2 diabetes. Dyslipidemia, on the other hand, plays a significant role in attracting activated immune cells such as macrophages, dendritic cells, T cells, NK cells, and B cells to visceral adipose tissue. These immune cells are a primary source of pro-inflammatory cytokines that are believed to promote insulin resistance. This review delves into the influence of elevated dietary free saturated fatty acids and examines the cellular and molecular factors associated with insulin resistance in the initiation of inflammation induced by obesity. Furthermore, it explores novel concepts related to diet-induced inflammation and its relationship with type 2 diabetes.
Full article
(This article belongs to the Section Innate Immunity and Inflammation)
►▼
Show Figures

Figure 1
Open AccessArticle
IL-12p40 Monomer: A Potential Player in Macrophage Regulation
by
Brian Jeong and Kalipada Pahan
Immuno 2024, 4(1), 77-90; https://doi.org/10.3390/immuno4010005 - 23 Feb 2024
Abstract
Macrophages are myeloid phagocytic leukocytes whose functions are to protect against infections, mediate T-cell responses, and maintain tissue homeostasis. IL-12p40 monomer is a cytokine that is largely produced by macrophages, and it has, for the longest time, been considered a largely non-functional cytokine
[...] Read more.
Macrophages are myeloid phagocytic leukocytes whose functions are to protect against infections, mediate T-cell responses, and maintain tissue homeostasis. IL-12p40 monomer is a cytokine that is largely produced by macrophages, and it has, for the longest time, been considered a largely non-functional cytokine of the IL-12 family. However, new research has emerged that demonstrates that this p40 monomer may play a bigger role in shaping immune environments. To shed light on the specific effects of p40 monomer on macrophages and their surrounding environment, we showed, through cell culture studies, qPCR, ELISA, and immunofluorescence analyses, that the direct administration of recombinant p40 monomer to RAW 264.7 cells and primary lung macrophages stimulated the production of both pro-inflammatory (TNFα) and anti-inflammatory (IL-10) signals. Accordingly, p40 monomer prevented the full pro-inflammatory effects of LPS, and the neutralization of p40 monomer by mAb a3-3a stimulated the pro-inflammatory effects of LPS. Furthermore, we demonstrated that the intranasal administration of p40 monomer upregulated TNFα+IL-10+ macrophages in vivo in the lungs of mice. Collectively, these results indicate an important immunoregulatory function of p40 monomer in the upregulation of both pro- and anti-inflammatory molecules in macrophages.
Full article
(This article belongs to the Section Autoimmunity and Immunoregulation)
►▼
Show Figures
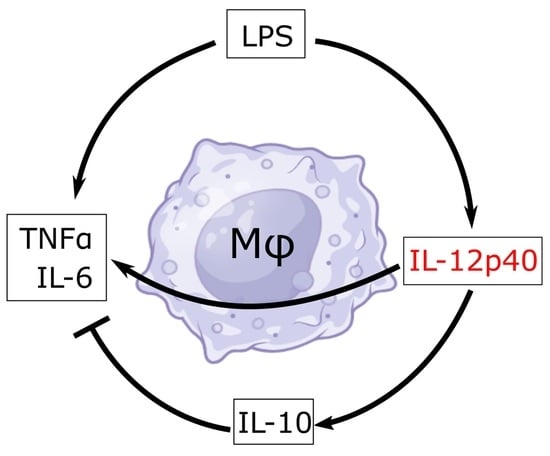
Graphical abstract
Open AccessReview
Immune Dysregulation and Current Targeted Biologics in Hidradenitis Suppurativa
by
Rene Chen, Robyn Guo, Amy J. Petty and Tarannum Jaleel
Immuno 2024, 4(1), 57-76; https://doi.org/10.3390/immuno4010004 - 02 Feb 2024
Abstract
►▼
Show Figures
Hidradenitis Suppurativa (HS) is a debilitating cutaneous disease characterized by a vicious cycle of chronic inflammation and tissue destruction that stems from disruption of the skin microbiome and abnormal activation of both the innate and adaptive immune system. A hallmark of HS pathophysiology
[...] Read more.
Hidradenitis Suppurativa (HS) is a debilitating cutaneous disease characterized by a vicious cycle of chronic inflammation and tissue destruction that stems from disruption of the skin microbiome and abnormal activation of both the innate and adaptive immune system. A hallmark of HS pathophysiology is dysregulation of both the innate and adaptive immune system. The role of immune system dysregulation in HS development has motivated researchers to explore the utility of biologic immunomodulators. In 2015, adalimumab, a tumor necrosis factor-α inhibitor, was approved by the Food and Drug Administration (FDA) for treatment of moderate-to-severe HS in the US. In 2023, secukinumab, an interleukin-17A (IL-17A) inhibitor, was approved by the European Medicines Agency for treatment of moderate-to-severe HS in Europe. Ongoing clinical trials have shown promising clinical responses to targeted therapies against other pro-inflammatory cytokines including IL-17, IL-12, IL-1, IL-36, IL-6, IL-10, interferon γ, C5a, and Janus kinase (JAK). We provide an update on the efficacy and clinical usage of targeted biologics in HS treatment.
Full article

Figure 1
Open AccessArticle
Markers for Immunological Resilience: Effects of Moderate- and High-Intensity Endurance Exercise on the Kinetic Response of Leukocyte Subsets
by
Shirley W. Kartaram, Marc Teunis, Klaske van Norren, Mieke Smits, Laura M’Rabet, Martie C. M. Verschuren, Karin Mohrmann, Johan Garssen, Renger Witkamp and Raymond Pieters
Immuno 2024, 4(1), 43-56; https://doi.org/10.3390/immuno4010003 - 30 Jan 2024
Abstract
The kinetic responses of leukocyte subsets to exercise and their recovery may serve as indicators of immunological resilience. These time-dependent responses were investigated in healthy young men using a bicycle ergometer test. Fifteen recreationally active male cyclists (20–35 years, VO2max 56.9 ±
[...] Read more.
The kinetic responses of leukocyte subsets to exercise and their recovery may serve as indicators of immunological resilience. These time-dependent responses were investigated in healthy young men using a bicycle ergometer test. Fifteen recreationally active male cyclists (20–35 years, VO2max 56.9 ± 3.9 mL kg−1 min−1) performed four exercise protocols with a 1 h duration in a cross-over design: at 70% of the maximal workload (Wmax) in a hydrated and a mildly dehydrated state, at 50% of the Wmax, and intermittently at 85/55% of the Wmax in blocks of 2 min. The numbers of lymphocytes, monocytes, neutrophils, eosinophils, basophils, thrombocytes, and NK cells (CD16 and CD56) were measured at different time points up to 24 h post-exercise. The total leukocyte counts and those of most subsets increased from the start of the exercise, peaking after 30–60 min of exercising. The neutrophil numbers, however, peaked 3 h post-exercise. The CD16brightCD56dim NK cells showed a 1.5-fold increase compared to the CD16brightCD56bright NK cells. Other than for MCP-1, no significant differences were found in the serum cytokine levels. Our results show that exercise intensity is reflected in different time-dependent changes in leukocyte subsets, which supports the concept that the exchange of immune cells between peripheral blood and tissues contributes to enhanced immune surveillance during strenuous exercise.
Full article
(This article belongs to the Section Clinical/translational Immunology)
►▼
Show Figures
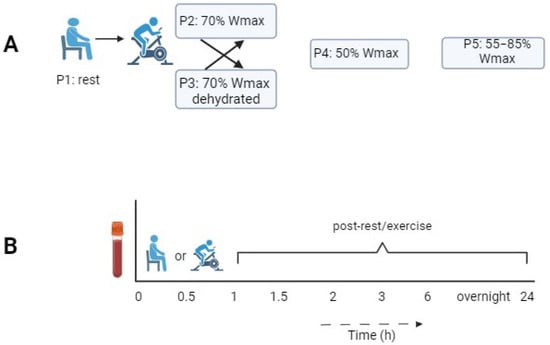
Figure 1
Open AccessReview
The Role of Structural Bioinformatics in Understanding Tumor Necrosis Factor α-Interacting Protein Mechanisms in Chronic Inflammatory Diseases: A Review
by
Luana Luiza Bastos, Diego Mariano, Rafael Pereira Lemos, Tatiane Senna Bialves, Carlo Jose Freire Oliveira and Raquel C. de Melo-Minardi
Immuno 2024, 4(1), 14-42; https://doi.org/10.3390/immuno4010002 - 15 Jan 2024
Abstract
Tumor necrosis factor α (TNF-α) is a multifunctional cytokine protein acknowledged as a vital mediator in cell differentiation, proliferation, and survival. Additionally, TNF-α is a crucial component of the host’s defense by mediating inflammatory and immune responses against various aggressive agents, including viruses,
[...] Read more.
Tumor necrosis factor α (TNF-α) is a multifunctional cytokine protein acknowledged as a vital mediator in cell differentiation, proliferation, and survival. Additionally, TNF-α is a crucial component of the host’s defense by mediating inflammatory and immune responses against various aggressive agents, including viruses, bacteria parasites, and tumors. However, excessive production can be detrimental to the body and is also implicated in developing several inflammatory and immune-mediated disorders. Therefore, there is great interest in studying its role and its modulation, in various diseases, both in in vitro, in vivo, and in silico experiments. In this review, we evaluated the structures of proteins related to TNF-α available in public databases. In addition, we described the main antibodies blocking this cytokine and its applications and commented on the potential of naturally produced binding molecules, such as TNF-α-binding proteins produced by ticks. We also discuss the role of structural bioinformatics techniques in understanding the mechanisms of chronic inflammatory diseases related to TNF-α. We hope that the data presented in this review will be useful for studies that aim to better understand the mechanisms of the interactions of TNF-α with other proteins and will lead to new drugs or treatments.
Full article
(This article belongs to the Section Structural Immunology)
►▼
Show Figures

Figure 1
Open AccessReview
Catastrophic Antiphospholipid Syndrome: A Review
by
Carmine Siniscalchi, Manuela Basaglia, Michele Riva, Michele Meschi, Tiziana Meschi, Giampiero Castaldo and Pierpaolo Di Micco
Immuno 2024, 4(1), 1-13; https://doi.org/10.3390/immuno4010001 - 25 Dec 2023
Cited by 1
Abstract
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by thrombotic or obstetric events occurring in individuals who have persistent antiphospholipid antibodies. Catastrophic antiphospholipid syndrome (CAPS) is a rare and potentially fatal form of APS characterized by severe thrombotic complications occurring in multiple
[...] Read more.
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterized by thrombotic or obstetric events occurring in individuals who have persistent antiphospholipid antibodies. Catastrophic antiphospholipid syndrome (CAPS) is a rare and potentially fatal form of APS characterized by severe thrombotic complications occurring in multiple organs over a short period of time or simultaneously. CAPS is associated with a high (50%) death rate. Infections, multi-organ failure, and cerebral and heart thrombosis represent the main complications of this syndrome. Generally, anticoagulants, glucocorticoids, therapeutic plasmapheresis (TPE), and intravenous immunoglobulin (IVIG) are used in combination for treatment. Multidisciplinary care involving different specialists from hematology, rheumatology, nephrology, infectious disease, critical care, and obstetrics is often required due to the complexity of the disease. Recent data emphasize the effectiveness of biologics such as anti-TNF-a monoclonal antibodies (adalimumab, certolizumab), anti-CD38 monoclonal antibody (daratumumab), BAFF/Blys inhibitor (belimumab), and BTK inhibitor (zanubrutinib) against CAPS. In order to understand the underlying causes of CAPS, one future possibility involves investigating and characterizing the hereditary and acquired risk factors associated with CAPS.
Full article
(This article belongs to the Special Issue Recent Advances in Antiphospholipid Syndrome)
►▼
Show Figures
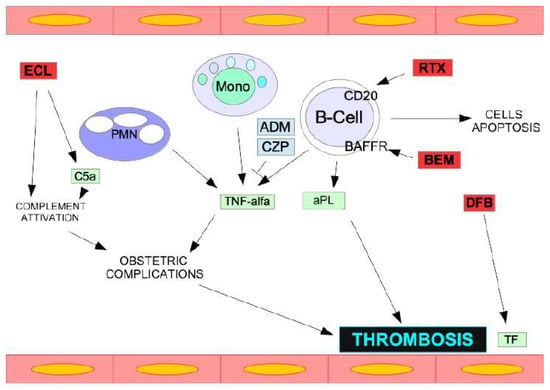
Figure 1
Open AccessReview
Inflammatory Network of Liver Fibrosis and How It Can Be Targeted Therapeutically
by
Kirstin O. Lowe, Constantin E. Tanase, Susan Maghami, Leanne E. Fisher and Amir M. Ghaemmaghami
Immuno 2023, 3(4), 375-408; https://doi.org/10.3390/immuno3040023 - 28 Nov 2023
Cited by 1
Abstract
Liver fibrosis is a complex, dynamic process associated with a broad spectrum of chronic liver diseases and acute liver failure, characterised by the dysregulated intrahepatic production of extracellular matrix proteins replacing functional liver cells with scar tissue. Fibrosis progresses due to an interrelated
[...] Read more.
Liver fibrosis is a complex, dynamic process associated with a broad spectrum of chronic liver diseases and acute liver failure, characterised by the dysregulated intrahepatic production of extracellular matrix proteins replacing functional liver cells with scar tissue. Fibrosis progresses due to an interrelated cycle of hepatocellular injury, triggering a persistent wound-healing response. The accumulation of scar tissue and chronic inflammation can eventually lead to cirrhosis and hepatocellular carcinoma. Currently, no therapies exist to directly treat or reverse liver fibrosis; hence, it remains a substantial global disease burden. A better understanding of the intricate inflammatory network that drives the initiation and maintenance of liver fibrosis to enable the rationale design of new intervention strategies is required. This review clarifies the most current understanding of the hepatic fibrosis cellular network with a focus on the role of regulatory T cells, and a possible trajectory for T cell immunotherapy in fibrosis treatment. Despite good progress in elucidating the role of the immune system in liver fibrosis, future work to better define the function of different immune cells and their mediators at different fibrotic stages is needed, which will enhance the development of new therapies.
Full article
(This article belongs to the Section Clinical/translational Immunology)
►▼
Show Figures
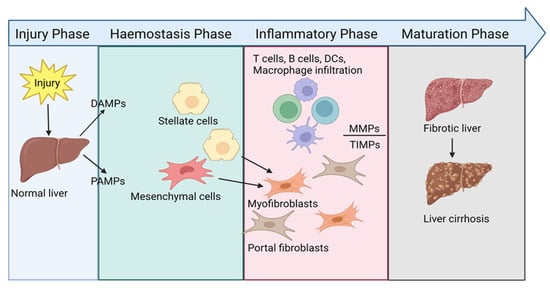
Figure 1
Open AccessArticle
High-Avidity Anti-Filovirus IgG Elicited Using Protein Subunit Vaccines Does Not Correlate with Protection
by
Caitlin A. Williams, Teri Ann S. Wong, Michael M. Lieberman, Jake Yalley-Ogunro, Mehtap Cabus, Sara Nezami, Fabian Paz, Hanne Andersen, Thomas W. Geisbert and Axel T. Lehrer
Immuno 2023, 3(4), 358-374; https://doi.org/10.3390/immuno3040022 - 24 Oct 2023
Abstract
Zaire ebolavirus (EBOV) poses a significant threat to public health due to its high case fatality rate and epidemic potential. This is further complicated by the lack of precise immune correlates of protection and difficulties in conducting in vivo animal studies due to
[...] Read more.
Zaire ebolavirus (EBOV) poses a significant threat to public health due to its high case fatality rate and epidemic potential. This is further complicated by the lack of precise immune correlates of protection and difficulties in conducting in vivo animal studies due to species specificity of Ebola virus disease (EVD) and classification as a biosafety level 4 pathogen. Related ebolaviruses have also contributed to the public health threat; Uganda recently experienced an outbreak of Sudan ebolavirus, which also had a high case fatality rate. Vaccination targeting EBOV has demonstrated significant efficacy; however, the protective cellular and humoral responses at play are still poorly understood. Vaccination for vulnerable populations such as pregnant women, young children, and immunocompromised individuals is still limited. Understanding vaccine correlates of protection (vCOP) is key to developing alternative vaccination strategies for these groups. Components of immunity such as neutralizing antibody and cell-mediated immunity are likely responsible for protective responses; however, existing research fails to fully define their roles in protection. Here we investigated vaccine-elicited antibody avidity as a potential correlate of protection and to further characterize the contribution of antibody avidity in protective and nonprotective vaccine responses.
Full article
(This article belongs to the Section Infectious Immunology and Vaccines)
►▼
Show Figures

Figure 1
Open AccessReview
Monoclonal War: The Antibody Arsenal and Targets for Expanded Application
by
Eric H. Rosenn, Mickael Benhaim, Allison Siegel, David A. Stein, Joseph S. Leonard, Erik Katcher, Dania Halperin and Zachary Mostel
Immuno 2023, 3(3), 346-357; https://doi.org/10.3390/immuno3030021 - 20 Sep 2023
Abstract
Advancements in sequencing and screening technology have made monoclonal antibodies more accessible, cost-effective, and precise. These drugs effectively target pathogens and cancer cells and even regulate metabolic pathways by focusing on specific intermediates. Monoclonal antibodies play a key role in mitigating a rise
[...] Read more.
Advancements in sequencing and screening technology have made monoclonal antibodies more accessible, cost-effective, and precise. These drugs effectively target pathogens and cancer cells and even regulate metabolic pathways by focusing on specific intermediates. Monoclonal antibodies play a key role in mitigating a rise in occupation-related cancers, neurodegenerative disorders, and multidrug-resistant organisms. Here, we review the origins, mechanisms, and applications of this important drug class and explore future avenues for research.
Full article
(This article belongs to the Section Synthetic Immunity and Immune Engineering)
►▼
Show Figures
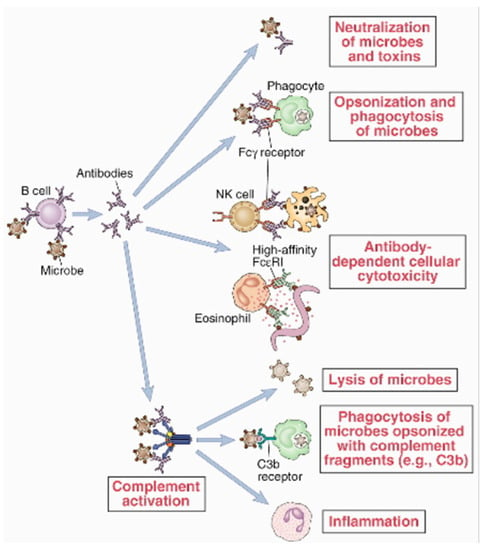
Figure 1
Open AccessArticle
Increased COVID-19 Mortality and Deficient SARS-CoV-2 Immune Response Are Not Associated with Higher Levels of Endemic Coronavirus Antibodies
by
Bindu Adhikari, Eugene M. Oltz, Joseph S. Bednash, Jeffrey C. Horowitz, Joshua O. Amimo, Sergei A. Raev, Soledad Fernández, Mirela Anghelina, Shan-Lu Liu, Mark P. Rubinstein, Daniel M. Jones, Linda J. Saif and Anastasia N. Vlasova
Immuno 2023, 3(3), 330-345; https://doi.org/10.3390/immuno3030020 - 04 Sep 2023
Cited by 1
Abstract
►▼
Show Figures
The impact of pre-existing common cold coronavirus (CCCoV) antibodies (Abs) on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immune responses and pathogenesis remains poorly defined. We evaluated these associations in a cohort of hospitalized patients with COVID-19 and respiratory failure of varying severity.
[...] Read more.
The impact of pre-existing common cold coronavirus (CCCoV) antibodies (Abs) on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immune responses and pathogenesis remains poorly defined. We evaluated these associations in a cohort of hospitalized patients with COVID-19 and respiratory failure of varying severity. Patients with respiratory failure from other causes (non-COVID-19) were evaluated as controls. We demonstrated a positive correlation between levels of CCCoV and SARS-CoV-2 Abs using CCCoV and SARS-CoV-2 N and S protein peptide-specific ELISA. Consistent with the above, moderately increased levels of CCCoV-specific Abs in non-COVID-19 vs. COVID-19 patients suggest potential protective effects. Further, higher SARS-CoV-2 N protein-specific and CCCoV Ab levels were observed among surviving vs. non-surviving COVID-19 positive patients. However, the highest SARS-CoV-2 N and S protein-specific IgG and IgA Ab levels were noted in the patients with the most severe clinical disease. Finally, advanced age, cancer and immunosuppression were associated with significantly higher mortality and reduced SARS-CoV-2 and CCCoV Ab levels. Thus, our data highlight that sufficient SARS-CoV-2 N protein-specific Ab responses improve clinical outcomes in severely ill COVID-19 patients. We also confirmed that pre-existing CCCoV-specific Abs do not inhibit the SARS-CoV-2 Ab response and may further reduce the prevalence and/or severity of COVID-19.
Full article

Figure 1
Open AccessReview
Bone Marrow: The Central Immune System
by
Volker Schirrmacher
Immuno 2023, 3(3), 289-329; https://doi.org/10.3390/immuno3030019 - 03 Aug 2023
Cited by 2
Abstract
Bone marrow is known as the site of hematopoiesis. What is not being described in textbooks of immunology is the fact that bone marrow is not only a generative, but also an antigen-responsive, immune organ. It is also a major storage site for
[...] Read more.
Bone marrow is known as the site of hematopoiesis. What is not being described in textbooks of immunology is the fact that bone marrow is not only a generative, but also an antigen-responsive, immune organ. It is also a major storage site for antigen-specific memory B and T cells. That bone marrow is a priming site for T cell responses to blood borne antigens was discovered exactly 20 years ago. This review celebrates this important discovery. The review provides a number of examples of medical relevance of bone marrow as a central immune system, including cancer, microbial infections, autoimmune reactions, and bone marrow transplantation. Bone marrow mesenchymal stem cell-derived stromal cells provide distinct bone marrow niches for stem cells and immune cells. By transmitting anti-inflammatory dampening effects, facilitating wound healing and tissue regeneration mesenchymal stem cells contribute to homeostasis of bone and other tissues. Based on the evidence presented, the review proposes that bone marrow is a multifunctional and protective immune system. In an analogy to the central nervous system, it is suggested that bone marrow be designated as the central immune system.
Full article
(This article belongs to the Section Cancer Immunology and Immunotherapy)
►▼
Show Figures
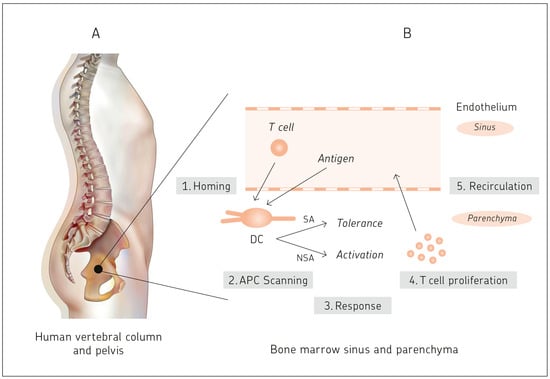
Figure 1
Open AccessArticle
CDX-585, a Bispecific Antibody with Dual Targeting of ILT4 and PD-1 Checkpoint Pathways
by
Michael B. Murphy, Laura Vitale, Shukai Xia, Zeyu Peng, Thomas O’Neill, Jay Lillquist, Anna Wasiuk, Jeff Weidlick, Jenifer Widger, Laura Mills-Chen, Andrea Crocker, Colleen Patterson, James Boyer, April R. Baronas, Mingjiu Chen, Hugh M. Davis, Mark Ma, Joel Goldstein, Lawrence J. Thomas, Diego Alvarado, Henry C. Marsh and Tibor Keleradd
Show full author list
remove
Hide full author list
Immuno 2023, 3(3), 273-288; https://doi.org/10.3390/immuno3030018 - 07 Jul 2023
Cited by 1
Abstract
Immunoglobulin-like transcript 4 (ILT4) is an immunosuppressive molecule predominantly expressed on myeloid cells. Recent studies combining ILT4 suppression with programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) blockade have shown promising signs of activity in immune checkpoint inhibitor refractory patients. We
[...] Read more.
Immunoglobulin-like transcript 4 (ILT4) is an immunosuppressive molecule predominantly expressed on myeloid cells. Recent studies combining ILT4 suppression with programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) blockade have shown promising signs of activity in immune checkpoint inhibitor refractory patients. We theorized that coupling ILT4 and PD-1/PD-L1 blockade in a bispecific antibody (bsAb) may provide greater immune activating properties than combining the individual mAbs due to enhanced bridging of APCs to T cells. To test this approach, we developed CDX-585, a tetravalent ILT4xPD-1 IgG1-scFv bsAb from novel PD-1 and ILT-4 mAbs. CDX-585 is a potent antagonist of both PD-1 and ILT4. CDX-585 promotes M1 macrophage polarization and enhances pro-inflammatory cytokine secretion in response to lipopolysaccharide or CD40 agonist mAb treatment. In mixed lymphocyte reaction (MLR) assays, CDX-585 is more potent than the combination of parental antibodies. In a humanized NCG mouse SK-MEL-5 tumor model, CDX-585 exhibits greater antitumor activity than the combination of parental mAbs. A pilot study of CDX-585 in cynomolgus macaques confirmed a mAb-like pharmacokinetic profile without noted toxicities. These studies demonstrate that CDX-585 effectively combines ILT4 and the PD-1 blockade into one molecule that is more potent than the combination of the parental antibodies, providing the rationale to advance this bsAb into clinical studies.
Full article
(This article belongs to the Section Cancer Immunology and Immunotherapy)
►▼
Show Figures
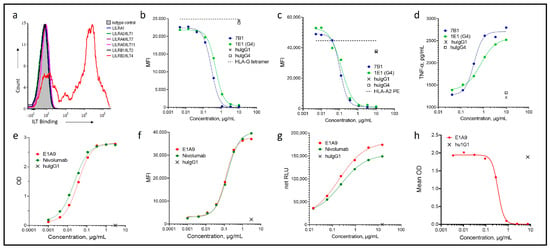
Figure 1
Open AccessReview
The Importance of Neutrophils in Osteoarthritis: Current Concepts and Therapeutic Perspectives
by
Yeganeh Mehrani, Rasool Rahimi Junqani, Solmaz Morovati, Hossein Mehrani, Negar Karimi and Samaneh Ghasemi
Immuno 2023, 3(3), 250-272; https://doi.org/10.3390/immuno3030017 - 05 Jul 2023
Cited by 2
Abstract
Osteoarthritis (OA) is the most common degenerative joint disease that causes chronic pain and disability. Different innate immune components, including macrophages, T cells, and neutrophils, participate in OA pathophysiology. Neutrophils are the most abundant circulating leukocytes with multiple specialized functions contributing to innate
[...] Read more.
Osteoarthritis (OA) is the most common degenerative joint disease that causes chronic pain and disability. Different innate immune components, including macrophages, T cells, and neutrophils, participate in OA pathophysiology. Neutrophils are the most abundant circulating leukocytes with multiple specialized functions contributing to innate and adaptive immune functions. Although neutrophils produce proinflammatory cytokines and chemokines, reactive oxygen species (ROS), matrix-degrading enzymes, and neutrophil extracellular traps (NET) that promote joint degradation as the first recruit cells in an inflamed joint, these cells also play an important role in joint repair by regulating the immune response, releasing anti-inflammatory factors, and activating certain protective genes. In this review, various aspects of neutrophil biology, their role in inflammation and its association with OA, and possible therapeutic approaches to target neutrophils for the treatment of OA are described. Since neutrophils play a complex role in the pathophysiology of osteoarthritis, contributing to joint degradation as well as joint repair, targeting these cells is likely to pave the way for a potential therapeutic approach for the management of OA. Future studies are needed to investigate the use of targeted therapies to modulate neutrophil function and identify their subpopulations that are associated with osteoarthritis progression or response to treatment.
Full article
(This article belongs to the Section Innate Immunity and Inflammation)
►▼
Show Figures
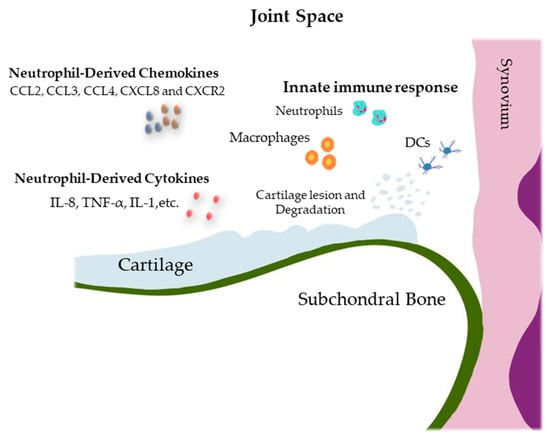
Figure 1
Open AccessArticle
Immunotherapeutic Development of a Tri-Specific NK Cell Engager Recognizing BCMA
by
Felix Oh, Martin Felices, Behiye Kodal, Jeffrey S. Miller and Daniel A. Vallera
Immuno 2023, 3(2), 237-249; https://doi.org/10.3390/immuno3020016 - 20 Jun 2023
Abstract
Chemotherapy-refractive multiple myeloma (MM) is serious and life-threatening, and better treatments are urgently needed. BCMA is a prominent marker on the cell surface of MM cells, rendering it an accepted target for antibody therapy. Considering that MM is a liquid tumor and immunotherapy
[...] Read more.
Chemotherapy-refractive multiple myeloma (MM) is serious and life-threatening, and better treatments are urgently needed. BCMA is a prominent marker on the cell surface of MM cells, rendering it an accepted target for antibody therapy. Considering that MM is a liquid tumor and immunotherapy has enjoyed success against leukemia, we devise an approach designed to enhance NK cell activity against MM. Ordinarily, NK cells function to naturally survey the body and eliminate malignant cells. Our platform approach is designed to enhance NK function. A tri-specific immune-engaging TriKE is manufactured, consisting of a camelid nanobody VHH antibody fragment recognizing CD16 expressed on NK cells and an scFv antibody fragment specifically recognizing BCMA. These two fragments are crosslinked by the human cytokine interleukin-15 (IL-15) known to have prominent activating effects on NK cells. The molecule, when tested by flow cytometry, shows activation of NK cells in their numbers and activity. Additionally, the molecule demonstrates anti-cancer effects in an in vivo xenograft model of human MM. We believe that the drug will have the capability of enhancing NK cells at the site of the immune synapse, i.e., the effector:target cell interface, and this will promote cancer remissions.
Full article
(This article belongs to the Section Cancer Immunology and Immunotherapy)
►▼
Show Figures

Figure 1
Open AccessReview
Inflammatory Profile of Th9 Cells and Their Protective Potential in Helminth Infections
by
Yvanna Louise Di Christine Oliveira, Yrna Lorena Matos de Oliveira, Tatyane Martins Cirilo, Ricardo Toshio Fujiwara, Lilian Lacerda Bueno and Silvio Santana Dolabella
Immuno 2023, 3(2), 228-236; https://doi.org/10.3390/immuno3020015 - 08 Jun 2023
Abstract
In terms of the global burden of disease, helminthiasis is the most common infectious disease in the world. In response to the disease, the human host develops an immunological response that occurs predominantly through the action of T helper 2 (Th2) cells and
[...] Read more.
In terms of the global burden of disease, helminthiasis is the most common infectious disease in the world. In response to the disease, the human host develops an immunological response that occurs predominantly through the action of T helper 2 (Th2) cells and the interleukins IL-4, IL-5 and IL-13. However, other types of Th cells, such as Th9, are also involved in the defense against helminths, with the IL-9 produced by these cells promoting the induction of mastocytosis and the increased production of IgG1 and IgE, in addition to the increase in intestinal contractility that promotes the expulsion of worms. Together, IL-9 and IL-10, which is also produced by Th9, induce a type 2 inflammatory response characterized by the coordinated actions of innate lymphoid cells, mast cells, basophils and other cells that work together toward a single objective: the reduction of the parasitic burden. This review presents the latest findings on Th9 effector mechanisms in helminthic infections.
Full article
(This article belongs to the Section Immunopathology and Immunohistology)
►▼
Show Figures

Figure 1
Open AccessReview
Exploring the Potential of Plant-Based CTB-INS Oral Vaccines in Treating Type 1 Diabetes
by
Jacques C. Mbongue, Elaine Vanterpool and William H. R. Langridge
Immuno 2023, 3(2), 217-227; https://doi.org/10.3390/immuno3020014 - 01 Jun 2023
Abstract
The 19th century saw the development of vaccines, which were biological preparations designed to enhance immunity against specific diseases. Edible vaccines function by stimulating both systemic and mucosal immune responses against foreign pathogens, and they may potentially protect the host from autoimmunity. The
[...] Read more.
The 19th century saw the development of vaccines, which were biological preparations designed to enhance immunity against specific diseases. Edible vaccines function by stimulating both systemic and mucosal immune responses against foreign pathogens, and they may potentially protect the host from autoimmunity. The mucosal surfaces provide a convenient and rapid route for delivering therapeutic small molecules. This is due to their large surface areas and easy administration. The effectiveness of mucosal immunization relies on the fact that mucous membranes represent the body’s largest immunogenic organ. Within this interface, there is a well-organized lymphatic structure known as MALT (mucosa-associated lymphoid tissue), which includes both T and B cells and encompasses the adaptive arms of the immune system. Oral vaccines specifically stimulate immune responses in the gut-associated lymphoid tissue (GALT), which consists of lymph nodes, Payer’s patches (where B cells make up about 75% of the population and T cells account for approximately 20%), and isolated lymphoid follicles within the gastrointestinal tract (GIT). However, a significant challenge in developing vaccines is the rapid degradation of antigens within the harsh environment of the digestive tract, which hampers effective protein delivery to the GIT. In light of recent proteomic analysis revealing strong up-regulation of the tryptophan catabolic enzyme indoleamine 2, 3-dioxygenase (IDO1) in DCs inoculated with the Cholera toxin B-subunit-Insulin fusion protein vaccine (CTB-INS), we are interested in investigating the effects of transgene integration into a selected plant cell as an edible vaccine.
Full article
(This article belongs to the Section Mucosal Immunology)
►▼
Show Figures
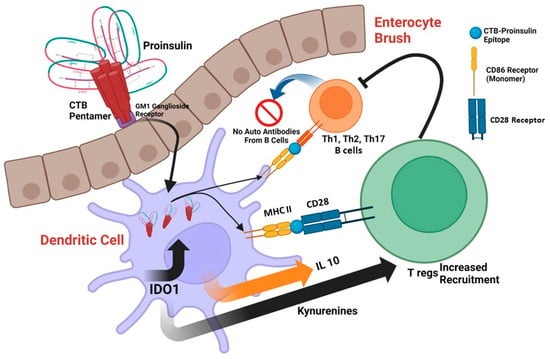
Figure 1
Open AccessReview
Current Advancements and Future Perspectives of Immunotherapy in Breast Cancer Treatment
by
Maria Vasileiou, Savvas Papageorgiou and Nam P. Nguyen
Immuno 2023, 3(2), 195-216; https://doi.org/10.3390/immuno3020013 - 30 May 2023
Abstract
Breast cancer is the most commonly diagnosed cancer in women and is a leading cause of cancer death in women worldwide. Despite the available treatment options, such as surgery, chemotherapy, radiotherapy, endocrine therapy and molecular targeted therapy, breast cancer treatment remains a challenge.
[...] Read more.
Breast cancer is the most commonly diagnosed cancer in women and is a leading cause of cancer death in women worldwide. Despite the available treatment options, such as surgery, chemotherapy, radiotherapy, endocrine therapy and molecular targeted therapy, breast cancer treatment remains a challenge. The advent of immunotherapy has revolutionized the treatment of breast cancer as it utilizes the host’s immune system to directly target tumor cells. In this literature review, we aim to summarize the recent advancements made in using immunotherapy for treating breast cancer patients. We discuss the different types of existing immunotherapies for breast cancer, including targeted therapy using monoclonal antibodies against breast cancer specific antigens and the use of immune checkpoint inhibitors to elicit an immune response against cancer cells. Finally, we consider the development of breast cancer vaccines that train the immune system to specifically recognize cancer cells and the future perspectives of immunotherapy for breast cancer.
Full article
(This article belongs to the Section Cancer Immunology and Immunotherapy)
►▼
Show Figures

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cells, Geriatrics, Immuno, IJMS, JCM
Inflammaging: The Immunology of Aging
Topic Editors: Juan Pablo de Rivero Vaccari, Alejandro Martín-MontalvoDeadline: 30 June 2024
Topic in
Antibodies, Cancers, Immuno, IJMS, Vaccines
Anti-Tumor Immune Responses 2.0
Topic Editors: Massimo Zollo, Renata GrifantiniDeadline: 31 August 2024
Topic in
BioMedInformatics, Cancers, Cells, Diagnostics, Immuno, IJMS
Inflammatory Tumor Immune Microenvironment
Topic Editors: William Cho, Anquan ShangDeadline: 15 March 2025

Conferences
Special Issues
Special Issue in
Immuno
Anti-inflammatory and Immunomodulatory Medicinal Plants and Their Derived Immunomodulators
Guest Editors: Bashar Saad, Badiaa LyoussiDeadline: 31 May 2024
Special Issue in
Immuno
Effects of Malnutrition of Immune Response
Guest Editor: Juan Bautista De SanctisDeadline: 30 June 2024
Special Issue in
Immuno
Next-Generation Cancer Immunotherapy
Guest Editor: Toshihiko TorigoeDeadline: 30 September 2024







