Sphingolipid Signaling in Health and Disease (Closed)
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Pharmacology".
Viewed by 58654Editor
Interests: sphingolipid signaling; prostaglandins; chronic inflammatory kidney diseases
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear colleagues,
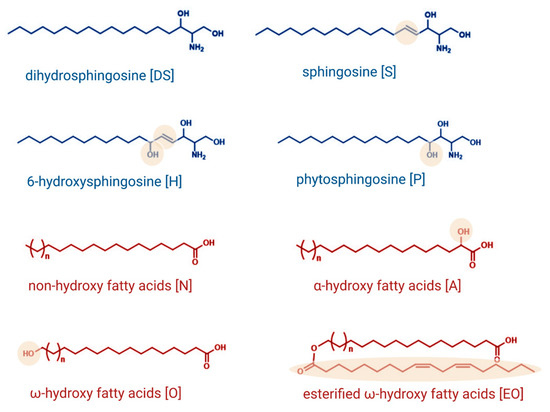
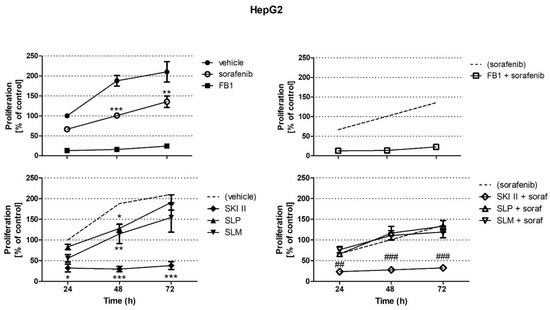
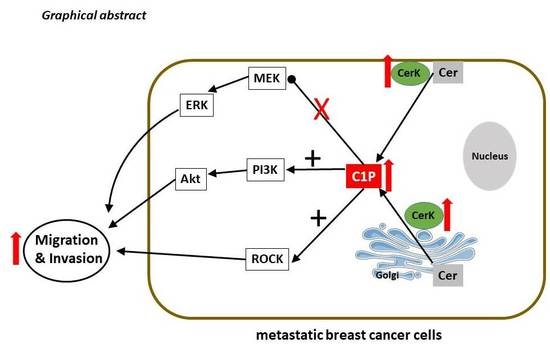
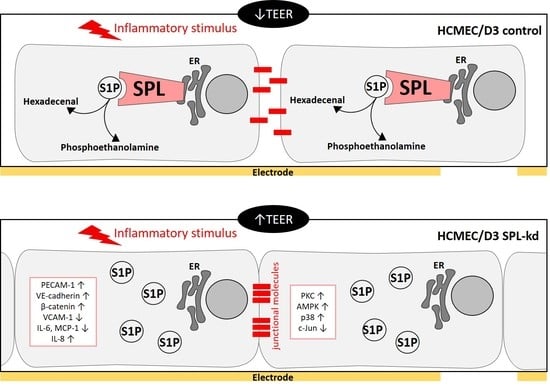
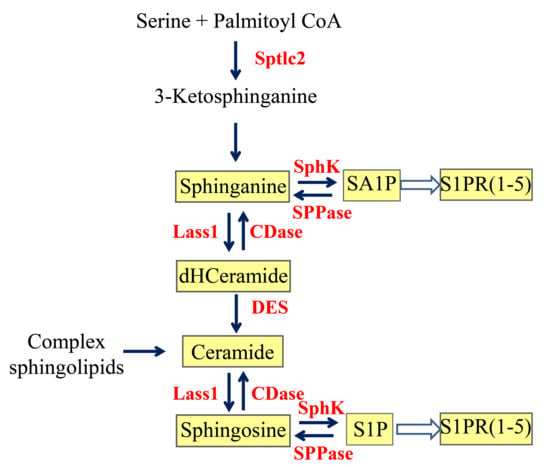
Sphingolipids have long been known as important structural components of biological membranes. This view has drastically changed after groundbreaking discoveries in the early 1990s, which showed that certain sphingolipid subspecies are bioactive and are able to induce signal transduction and regulate fundamental cellular responses such as cell proliferation and survival, differentiation, apoptosis, and migration. Evidence is now increasing that the dysregulation of sphingolipid signaling contributes to the pathogenesis of multiple diseases, including inflammation, pain, cancer, diabetes, cardiovascular dysfunction, organ fibrosis, and various neurodegenerative diseases. Nevertheless, the exact molecular mechanisms used by sphingolipids to mediate the cellular effects are still not completely understood. Also, the regulation of those enzymes that catalyze the generation and interconversion of sphingolipids, although being cloned and known for decades, are not well described.
This Topical Collection is dedicated to shedding light on the molecular mechanisms used by bioactive sphingolipids, including sphingosine, ceramides, glycosphingolipids, and phosphorylated sphingoids, and to better characterize their involvement in physiological, pathophysiological, and pathological conditions. Furthermore, advances in the development of new drugs and strategies to manipulate sphingolipid-regulating enzymes and receptors for therapeutic applications are welcome.
Prof. Andrea Huwiler
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- sphingolipids
- ceramide
- sphingosine-1-phosphate
- glycosphingolipids
- disease
- physiology