Oral Inflammation—Environment, Molecules, and Cells in Oral Physiology and Pathology
(Closed)
Share This Topical Collection
Editor
 Prof. Dr. Peter Proff
Prof. Dr. Peter Proff
 Prof. Dr. Peter Proff
Prof. Dr. Peter Proff
E-Mail
Guest Editor
Department of Orthodontics, Faculty of Medicine, University of Regensburg, Franz-Josef-Strauss-Allee 11, 93053 Regensburg, Germany
Interests: craniofacial development; orthodontic tooth movement; periodontal inflammation; oral immunology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Special Issue “Oral inflammation – Environment, Molecules, and Cells in Oral Physiology and Pathology” will cover a selection of recent research topics in the field of oral inflammation and immunology. Deciphering oral inflammatory processes and immune responses can advance our understanding of oral physiology and pathology, in general, with newly identified immune checkpoints being potential novel therapeutic targets for dental diseases and malformations.
The scope of this Special Issue, therefore, encompasses papers dealing with oral inflammation in etiopathogenesis and the clinical-biological manifestation of oral disease, malformations, and health with a focus on environment signals and molecules that can induce, shape, mediate, or propagate inflammatory conditions in the oral cavity, as well as on immune and other oral immune-supporting cells and their role in oral inflammatory responses.
Prof. Dr. Peter Proff
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
There is an Article Processing Charge (APC) for publication in this
open access journal. For details about the APC please see here.
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Oral inflammation
- Oral immunology
- Oral malformations
- Periodontitis
- Environment signals
- Cellular signaling
- Imaging
Related Special Issue
Published Papers (22 papers)
Open AccessArticle
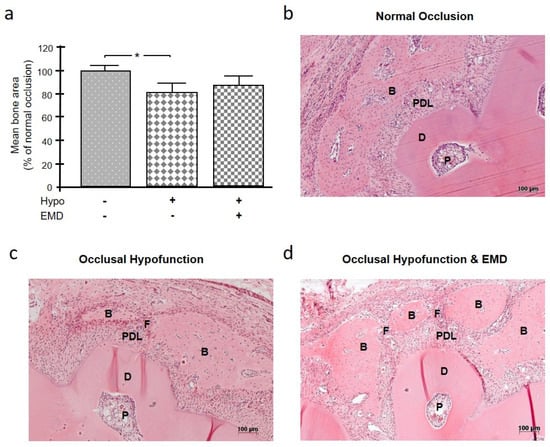
Influence of Occlusal Hypofunction on Alveolar Bone Healing in Rats
by
Anna Damanaki, Svenja Beisel-Memmert, Marjan Nokhbehsaim, Ali Abedi, Birgit Rath-Deschner, Andressa V. B. Nogueira and James Deschner
Viewed by 1139
Abstract
The aim of this in vivo study was to investigate the effect of occlusal hypofunction on alveolar bone healing in the absence or presence of an enamel matrix derivative (EMD). A standardized fenestration defect over the root of the mandibular first molar in
[...] Read more.
The aim of this in vivo study was to investigate the effect of occlusal hypofunction on alveolar bone healing in the absence or presence of an enamel matrix derivative (EMD). A standardized fenestration defect over the root of the mandibular first molar in 15 Wistar rats was created. Occlusal hypofunction was induced by extraction of the antagonist. Regenerative therapy was performed by applying EMD to the fenestration defect. The following three groups were established: (a) normal occlusion without EMD treatment, (b) occlusal hypofunction without EMD treatment, and (c) occlusal hypofunction with EMD treatment. After four weeks, all animals were sacrificed, and histological (hematoxylin and eosin, tartrate-resistant acid phosphatase) as well as immunohistochemical analyses (periostin, osteopontin, osteocalcin) were performed. The occlusal hypofunction group showed delayed bone regeneration compared to the group with normal occlusion. The application of EMD could partially, but not completely, compensate for the inhibitory effects of occlusal hypofunction on bone healing, as evidenced by hematoxylin and eosin and immunohistochemistry for the aforementioned molecules. Our results suggest that normal occlusal loading, but not occlusal hypofunction, is beneficial to alveolar bone healing. Adequate occlusal loading appears to be as advantageous for alveolar bone healing as the regenerative potential of EMD.
Full article
►▼
Show Figures
Open AccessArticle
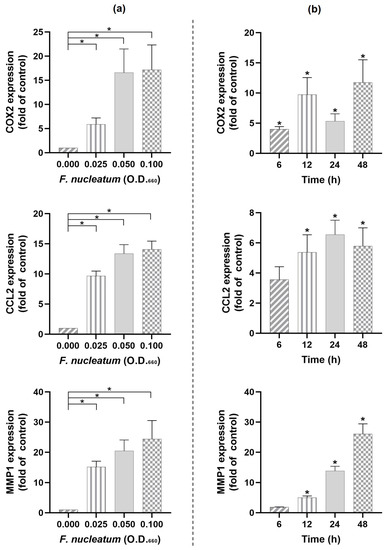
Apelin Enhances the Effects of Fusobacterium nucleatum on Periodontal Ligament Cells In Vitro
by
Pablo Cores Ziskoven, Andressa V. B. Nogueira, Lorena S. Gutierrez, Jens Weusmann, Sigrun Eick, Nurcan Buduneli and James Deschner
Cited by 1 | Viewed by 1180
Abstract
This study aimed to explore effects of
Fusobacterium nucleatum with or without apelin on periodontal ligament (PDL) cells to better understand pathomechanistic links between periodontitis and obesity. First, the actions of
F. nucleatum on COX2, CCL2, and MMP1 expressions were assessed. Subsequently, PDL
[...] Read more.
This study aimed to explore effects of
Fusobacterium nucleatum with or without apelin on periodontal ligament (PDL) cells to better understand pathomechanistic links between periodontitis and obesity. First, the actions of
F. nucleatum on COX2, CCL2, and MMP1 expressions were assessed. Subsequently, PDL cells were incubated with
F. nucleatum in the presence and absence of apelin to study the modulatory effects of this adipokine on molecules related to inflammation and hard and soft tissue turnover. Regulation of apelin and its receptor (APJ) by
F. nucleatum was also studied.
F. nucleatum resulted in elevated COX2, CCL2, and MMP1 expressions in a dose- and time-dependent manner. Combination of
F. nucleatum and apelin led to the highest (
p < 0.05) expression levels of COX2, CCL2, CXCL8, TNF-α, and MMP1 at 48 h. The effects of
F. nucleatum and/or apelin on CCL2 and MMP1 were MEK1/2- and partially NF-κB-dependent. The combined effects of
F. nucleatum and apelin on CCL2 and MMP1 were also observed at protein level. Moreover,
F. nucleatum downregulated (
p < 0.05) the apelin and APJ expressions. In conclusion, obesity could contribute to periodontitis through apelin. The local production of apelin/APJ in PDL cells also suggests a role of these molecules in the pathogenesis of periodontitis.
Full article
►▼
Show Figures
Open AccessArticle
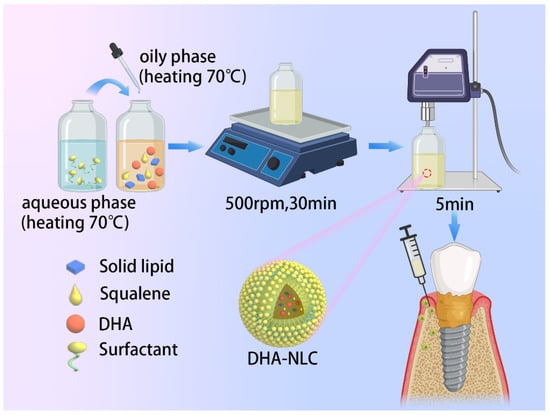
Docosahexaenoic Acid-Loaded Nanostructured Lipid Carriers for the Treatment of Peri-Implantitis in Rats
by
Zhen Li, Zhaoyi Yin, Baosheng Li, Jie He, Yanqun Liu, Ni Zhang, Xiaoyu Li, Qing Cai and Weiyan Meng
Cited by 4 | Viewed by 1819
Abstract
Being the most common cause of implant failure, peri-implantitis is defined as a pathological condition associated with the occurrence of peri-implant plaque, characterized by peri-implant mucosal inflammation and progressive loss of the supporting bone tissue attributed to the persistence of pro-inflammatory cytokines. Docosahexaenoic
[...] Read more.
Being the most common cause of implant failure, peri-implantitis is defined as a pathological condition associated with the occurrence of peri-implant plaque, characterized by peri-implant mucosal inflammation and progressive loss of the supporting bone tissue attributed to the persistence of pro-inflammatory cytokines. Docosahexaenoic acid (DHA), which is a type of omega-3 polyunsaturated fatty acid, is generally used for the treatment of many inflammatory diseases. However, a suitable form for dosing and its therapeutic effect on peri-implantitis remain unclear. In this study, a novel nanostructured lipid carrier (NLC) loaded with squalene and DHA was fabricated (DHA-loaded NLC). The encapsulation efficiency and drug loading efficiency values of the DHA-loaded NLC were 78.13% ± 1.85% and 28.09% ± 0.48%, respectively. The release of DHA was gradual and steady until 144 h. In addition, the free-radical-scavenging rate of DHA-loaded NLC (0.57 ± 0.03) was much higher than that of sole DHA (0.17 ± 0.003). By inhibiting nuclear factor-κB p65 nuclear translocation, DHA-loaded NLC prevented the activation of nuclear factor-κB downstream inflammatory pathways and exerted anti-inflammatory effects on macrophages. Moreover, DHA-loaded NLC showed better effects on preventing alveolar bone resorption of rat peri-implantitis model than sole DHA. Hence, DHA-loaded NLC enhanced the anti-inflammatory bioavailability of DHA, offering a novel approach for the treatment of peri-implantitis.
Full article
►▼
Show Figures
Open AccessArticle
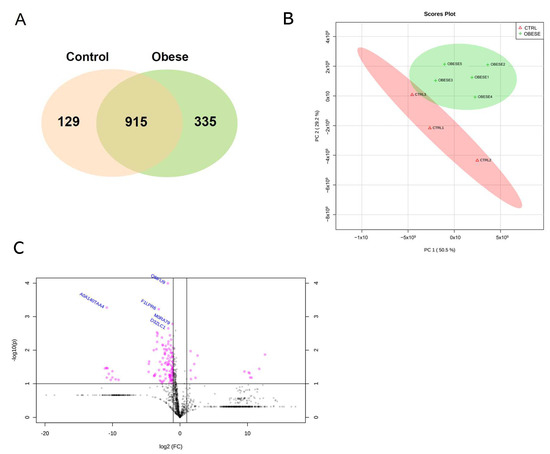
Obesity Modifies the Proteomic Profile of the Periodontal Ligament
by
Andressa V. B. Nogueira, Maria Eduarda S. Lopes, Camila C. Marcantonio, Cristiane R. Salmon, Luciana S. Mofatto, James Deschner, Francisco H. Nociti-Junior and Joni A. Cirelli
Viewed by 1210
Abstract
This study aimed to assess the obesity effects on the proteomic profile of the periodontal ligament of rats submitted to obesity induction by a high-fat diet. Eight Holtzman rats were divided into control (n = 3) and obese (n = 5) groups. The
[...] Read more.
This study aimed to assess the obesity effects on the proteomic profile of the periodontal ligament of rats submitted to obesity induction by a high-fat diet. Eight Holtzman rats were divided into control (n = 3) and obese (n = 5) groups. The maxillae were histologically processed for laser capture microdissection of the periodontal ligament of the first maxillary molars. Peptide mixtures were analyzed by LC-MS/MS. A total of 1379 proteins were identified in all groups. Among them, 335 (24.30%) were exclusively detected in the obese group, while 129 (9.35%) proteins were uniquely found in the control group. Out of the 110 (7.98%) differentially abundant proteins, 10 were more abundant and 100 had decreased abundance in the obese group. A gene ontology analysis showed some proteins related to obesity in the “extracellular exosome” term among differentially identified proteins in the gene ontology cellular component terms Prelp, Sec13, and Sod2. These three proteins were upregulated in the obese group (
p < 0.05), as shown by proteomic and immunohistochemistry analyses. In summary, our study presents novel evidence that the proteomic profile of the periodontal ligament is altered in experimental obesity induction, providing a list of differentially abundant proteins associated with obesity, which indicates that the periodontal ligament is responsive to obesity.
Full article
►▼
Show Figures
Open AccessArticle
Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase
by
Yüksel Korkmaz, Markus Plomann, Behrus Puladi, Aysegül Demirbas, Wilhelm Bloch and James Deschner
Viewed by 1802
Abstract
The binding of nitric oxide (NO) to heme in the β
1 subunit of soluble guanylyl cyclase (sGC) activates both the heterodimeric α
1β
1 and α
2β
1 isoforms of the enzyme, leading to the increased production of cGMP from
[...] Read more.
The binding of nitric oxide (NO) to heme in the β
1 subunit of soluble guanylyl cyclase (sGC) activates both the heterodimeric α
1β
1 and α
2β
1 isoforms of the enzyme, leading to the increased production of cGMP from GTP. In cultured human mast cells, exogenous NO is able to inhibit mast cell degranulation via NO-cGMP signaling. However, under inflammatory oxidative or nitrosative stress, sGC becomes insensitive to NO. The occurrence of mast cells in healthy and inflamed human tissues and the in vivo expression of the α
1 and β
1 subunits of sGC in human mast cells during inflammation remain largely unresolved and were investigated here. Using peroxidase and double immunohistochemical incubations, no mast cells were found in healthy dental pulp, whereas the inflammation of dental pulp initiated the occurrence of several mast cells expressing the α
1 and β
1 subunits of sGC. Since inflammation-induced oxidative and nitrosative stress oxidizes Fe
2+ to Fe
3+ in the β
1 subunit of sGC, leading to the desensitization of sGC to NO, we hypothesize that the NO- and heme-independent pharmacological activation of sGC in mast cells may be considered as a regulatory strategy for mast cell functions in inflamed human dental pulp.
Full article
►▼
Show Figures
Open AccessArticle
Lipoteichoic Acid and Lipopolysaccharides Are Affected by p38 and Inflammatory Markers and Modulate Their Promoting and Inhibitory Effects on Osteogenic Differentiation
by
Kiyohide Ishihata, Chang-Hwan Seong, Toshiro Kibe, Kenta Nakazono, Fredy Mardiyantoro, Ryohei Tada, Masahiro Nishimura, Tetsuya Matsuguchi and Norifumi Nakamura
Cited by 3 | Viewed by 1647
Abstract
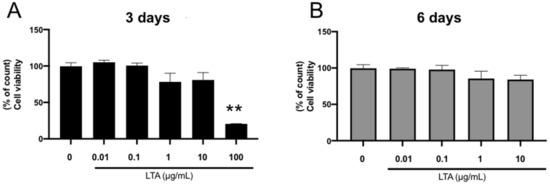
Lipoteichoic acid (LTA) and lipopolysaccharide (LPS) are cell wall components of Gram-positive and Gram-negative bacteria, respectively. Notably, oral microflora consists of a variety of bacterial species, and osteomyelitis of the jaw caused by dental infection presents with symptoms of bone resorption and osteosclerosis.
[...] Read more.
Lipoteichoic acid (LTA) and lipopolysaccharide (LPS) are cell wall components of Gram-positive and Gram-negative bacteria, respectively. Notably, oral microflora consists of a variety of bacterial species, and osteomyelitis of the jaw caused by dental infection presents with symptoms of bone resorption and osteosclerosis. However, the effects of LTA and LPS on osteogenic differentiation have not yet been clarified. We examined the effects of LTA and LPS on osteoblasts and found that LTA alone promoted alizarin red staining at low concentrations and inhibited it at high concentrations. Additionally, gene expression of osteogenic markers (ALP, OCN, and OPG) were enhanced at low concentrations of LTA. High concentrations of LPS suppressed calcification potential, and the addition of low concentrations of LTA inhibited calcification suppression, restoring the gene expression levels of suppressed bone differentiation markers (ALP, BSP, and OCN). Moreover, the suppression of p38, a signaling pathway associated with bone differentiation, had opposing effects on gene-level expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), suggesting that mixed LTA and LPS infections have opposite effects on bone differentiation through concentration gradients, involving inflammatory markers (TNF-α and IL-6) and the p38 pathway.
Full article
►▼
Show Figures
Open AccessArticle
Impact of Leptin on the Expression Profile of Macrophages during Mechanical Strain In Vitro
by
Eva Paddenberg, Hannah Osterloh, Jonathan Jantsch, Andressa Nogueira, Peter Proff, Christian Kirschneck and Agnes Schröder
Viewed by 1606
Abstract
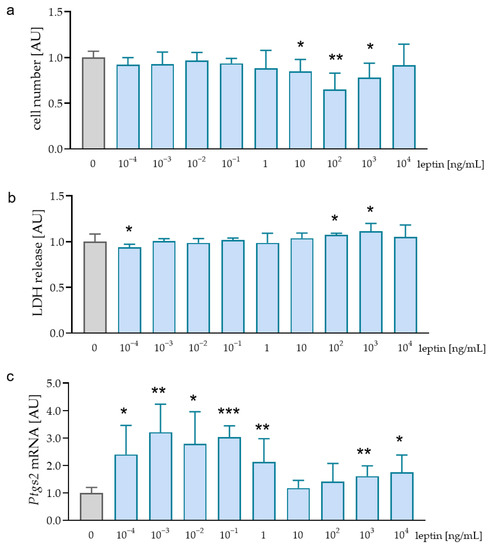
Childhood obesity is a growing problem in industrial societies and associated with increased leptin levels in serum and salvia. Orthodontic treatment provokes pressure and tension zones within the periodontal ligament, where, in addition to fibroblasts, macrophages are exposed to these mechanical loadings. Given
[...] Read more.
Childhood obesity is a growing problem in industrial societies and associated with increased leptin levels in serum and salvia. Orthodontic treatment provokes pressure and tension zones within the periodontal ligament, where, in addition to fibroblasts, macrophages are exposed to these mechanical loadings. Given the increasing number of orthodontic patients with these conditions, insights into the effects of elevated leptin levels on the expression profile of macrophages during mechanical strain are of clinical interest. Therefore, the aim of this in vitro study was to assess the influence of leptin on the expression profile of macrophages during simulated orthodontic treatment. RAW264.7 macrophages were incubated with leptin and lipopolysaccharides (LPS) from
Porphyromonas gingivalis (
P. gingivalis) or with leptin and different types of mechanical strain (tensile, compressive strain). Expression of inflammatory mediators including tumor necrosis factor (TNF), Interleukin-1-B (IL1B), IL6, and prostaglandin endoperoxide synthase (PTGS2) was assessed by RT-qPCR, ELISAs, and immunoblot. Without additional mechanical loading, leptin increased
Tnf,
Il1b,
Il6, and
Ptgs2 mRNA in RAW264.7 macrophages by itself and after stimulation with LPS. However, in combination with tensile or compressive strain, leptin reduced the expression and secretion of these inflammatory factors. By itself and in combination with LPS from
P. gingivalis, leptin has a pro-inflammatory effect. Both tensile and compressive strain lead to increased expression of inflammatory genes. In contrast to its effect under control conditions or after LPS treatment, leptin showed an anti-inflammatory phenotype after mechanical stress.
Full article
►▼
Show Figures
Open AccessArticle
RNA Sequencing Reveals the Upregulation of FOXO Signaling Pathway in Porphyromonas gingivalis Persister-Treated Human Gingival Epithelial Cells
by
Chuan Wang, Xuan Li, Tianfan Cheng, Leilei Wang and Lijian Jin
Cited by 3 | Viewed by 1955
Abstract
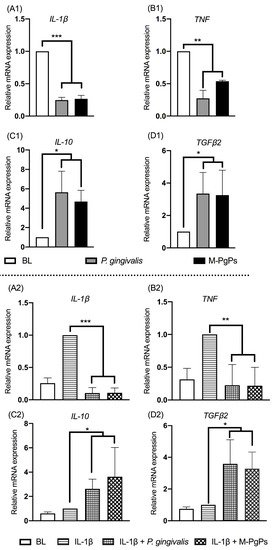
Porphyromonas gingivalis as the keystone periodontopathogen plays a critical role in the pathogenesis of periodontitis, and crucially accounts for inflammatory comorbidities such as cardiovascular disease and Alzheimer′s disease. We recently identified the existence of
P. gingivalis persisters and revealed the unforeseen perturbation of
[...] Read more.
Porphyromonas gingivalis as the keystone periodontopathogen plays a critical role in the pathogenesis of periodontitis, and crucially accounts for inflammatory comorbidities such as cardiovascular disease and Alzheimer′s disease. We recently identified the existence of
P. gingivalis persisters and revealed the unforeseen perturbation of innate response in human gingival epithelial cells (HGECs) due to these noxious persisters. Herein, RNA sequencing revealed how
P. gingivalis persisters affected the expression profile of cytokine genes and related signaling pathways in HGECs. Results showed that metronidazole-treated
P. gingivalis persisters (M-PgPs) impaired the innate host defense of HGECs, in a similar fashion to
P. gingivalis. Notably, over one thousand differentially expressed genes were identified in HGECs treated with M-PgPs or
P. gingivalis with reference to the controls. Gene Ontology and KEGG pathway analysis demonstrated significantly enriched signaling pathways, such as FOXO. Importantly, the FOXO1 inhibitor rescued the M-PgP-induced disruption of cytokine expression. This study suggests that
P. gingivalis persisters may perturb innate host defense, through the upregulation of the FOXO signaling pathway. Thus, the current findings could contribute to developing new approaches to tackling
P. gingivalis persisters for the effective control of periodontitis and
P. gingivalis-related inflammatory comorbidities.
Full article
►▼
Show Figures
Open AccessReview
Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement
by
Masaru Yamaguchi and Shinichi Fukasawa
Cited by 65 | Viewed by 9203
Abstract
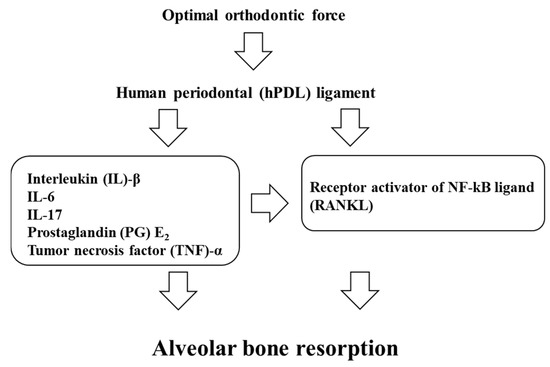
The aim of this paper is to provide a review on the role of inflammation in orthodontically induced inflammatory root resorption (OIIRR) and accelerating orthodontic tooth movement (AOTM) in orthodontic treatment. Orthodontic tooth movement (OTM) is stimulated by remodeling of the periodontal ligament
[...] Read more.
The aim of this paper is to provide a review on the role of inflammation in orthodontically induced inflammatory root resorption (OIIRR) and accelerating orthodontic tooth movement (AOTM) in orthodontic treatment. Orthodontic tooth movement (OTM) is stimulated by remodeling of the periodontal ligament (PDL) and alveolar bone. These remodeling activities and tooth displacement are involved in the occurrence of an inflammatory process in the periodontium, in response to orthodontic forces. Inflammatory mediators such as prostaglandins (PGs), interleukins (Ils; IL-1, -6, -17), the tumor necrosis factor (TNF)-α superfamily, and receptor activator of nuclear factor (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) are increased in the PDL during OTM. OIIRR is one of the accidental symptoms, and inflammatory mediators have been detected in resorbed roots, PDL, and alveolar bone exposed to heavy orthodontic force. Therefore, these inflammatory mediators are involved with the occurrence of OIIRR during orthodontic tooth movement. On the contrary, regional accelerating phenomenon (RAP) occurs after fractures and surgery such as osteotomies or bone grafting, and bone healing is accelerated by increasing osteoclasts and osteoblasts. Recently, tooth movement after surgical procedures such as corticotomy, corticision, piezocision, and micro-osteoperforation might be accelerated by RAP, which increases the bone metabolism. Therefore, inflammation may be involved in accelerated OTM (AOTM). The knowledge of inflammation during orthodontic treatment could be used in preventing OIIRR and AOTM.
Full article
►▼
Show Figures
Open AccessReview
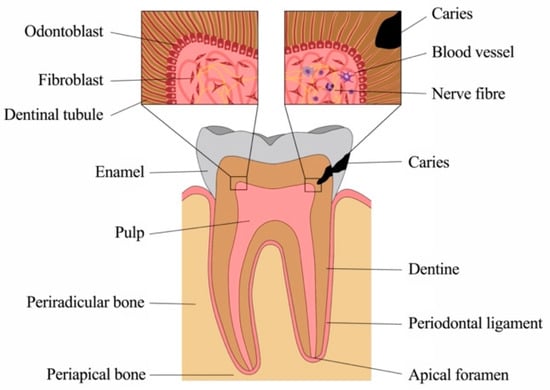
Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues
by
Kerstin M. Galler, Manuel Weber, Yüksel Korkmaz, Matthias Widbiller and Markus Feuerer
Cited by 131 | Viewed by 15748
Abstract
The macroscopic and microscopic anatomy of the oral cavity is complex and unique in the human body. Soft-tissue structures are in close interaction with mineralized bone, but also dentine, cementum and enamel of our teeth. These are exposed to intense mechanical and chemical
[...] Read more.
The macroscopic and microscopic anatomy of the oral cavity is complex and unique in the human body. Soft-tissue structures are in close interaction with mineralized bone, but also dentine, cementum and enamel of our teeth. These are exposed to intense mechanical and chemical stress as well as to dense microbiologic colonization. Teeth are susceptible to damage, most commonly to caries, where microorganisms from the oral cavity degrade the mineralized tissues of enamel and dentine and invade the soft connective tissue at the core, the dental pulp. However, the pulp is well-equipped to sense and fend off bacteria and their products and mounts various and intricate defense mechanisms. The front rank is formed by a layer of odontoblasts, which line the pulp chamber towards the dentine. These highly specialized cells not only form mineralized tissue but exert important functions as barrier cells. They recognize pathogens early in the process, secrete antibacterial compounds and neutralize bacterial toxins, initiate the immune response and alert other key players of the host defense. As bacteria get closer to the pulp, additional cell types of the pulp, including fibroblasts, stem and immune cells, but also vascular and neuronal networks, contribute with a variety of distinct defense mechanisms, and inflammatory response mechanisms are critical for tissue homeostasis. Still, without therapeutic intervention, a deep carious lesion may lead to tissue necrosis, which allows bacteria to populate the root canal system and invade the periradicular bone via the apical foramen at the root tip. The periodontal tissues and alveolar bone react to the insult with an inflammatory response, most commonly by the formation of an apical granuloma. Healing can occur after pathogen removal, which is achieved by disinfection and obturation of the pulp space by root canal treatment. This review highlights the various mechanisms of pathogen recognition and defense of dental pulp cells and periradicular tissues, explains the different cell types involved in the immune response and discusses the mechanisms of healing and repair, pointing out the close links between inflammation and regeneration as well as between inflammation and potential malignant transformation.
Full article
►▼
Show Figures
Open AccessArticle
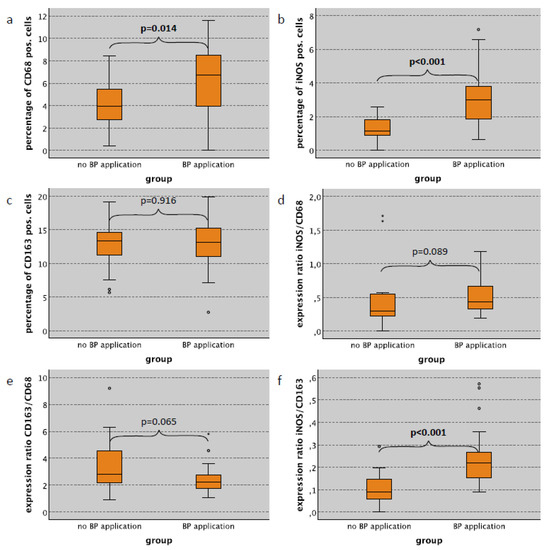
Zoledronate Causes a Systemic Shift of Macrophage Polarization towards M1 In Vivo
by
Manuel Weber, Andi Homm, Stefan Müller, Silke Frey, Kerstin Amann, Jutta Ries, Carol Geppert, Raimund Preidl, Tobias Möst, Peer W. Kämmerer, Marco Kesting and Falk Wehrhan
Cited by 16 | Viewed by 2825
Abstract
Background: Immunomodulatory properties of bisphosphonates (BP) are suggested to contribute to the development of medication-associated osteonecrosis of the jaw (MRONJ). Furthermore, bisphosphonate-derived immune modulation might contribute to the anti-metastatic effect observed in breast cancer patients. Macrophages are potential candidates for the mediation of
[...] Read more.
Background: Immunomodulatory properties of bisphosphonates (BP) are suggested to contribute to the development of medication-associated osteonecrosis of the jaw (MRONJ). Furthermore, bisphosphonate-derived immune modulation might contribute to the anti-metastatic effect observed in breast cancer patients. Macrophages are potential candidates for the mediation of immunomodulatory effects of bisphosphonates. The study aimed to investigate the influence of bisphosphonates alone and in combination with surgical trauma on systemic macrophage polarization (M1 vs. M2) using an in vivo rat model. Methods: A total of 120 animals were divided into four groups. Groups 2 and 4 were treated with 8 × 40 μg/kg body weight of the BP Zoledronate i.p. (week 0–7). Groups 3 and 4 were exposed to surgical trauma (week 8, tooth extraction + tibia fracture), whereas in Group 1 neither medication nor surgical trauma was applied. After 8, 10, 12 and 16 weeks, skin, lung and spleen were immunohistochemically examined for macrophage polarization via expression analysis of CD68, CD163 and iNOS using a tissue microarray (TMA). Results: A significant shift of macrophage polarization towards M1 was observed in skin, spleen and lung tissue of animals, with and without surgical trauma, treated with BP when compared to those without BP application. Surgical trauma did not cause a significant increase towards M1 polarization. Conclusions: BP application leads to a systemic pro-inflammatory situation in vivo, independent of surgical trauma, as evidenced by the shift in macrophage polarization towards M1 in various somatic tissues. This provides a possible explanation for the clinically observed anti-tumor effect of bisphosphonates and might also contribute to pathogenesis of MRONJ.
Full article
►▼
Show Figures
Open AccessReview
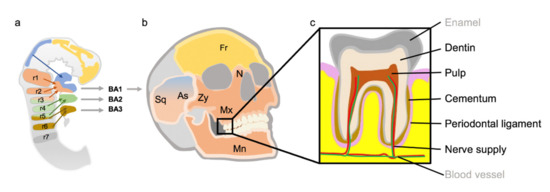
The Special Developmental Biology of Craniofacial Tissues Enables the Understanding of Oral and Maxillofacial Physiology and Diseases
by
Manuel Weber, Falk Wehrhan, James Deschner, Janina Sander, Jutta Ries, Tobias Möst, Aline Bozec, Lina Gölz, Marco Kesting and Rainer Lutz
Cited by 5 | Viewed by 2944
Abstract
Maxillofacial hard tissues have several differences compared to bones of other localizations of the human body. These could be due to the different embryological development of the jaw bones compared to the extracranial skeleton. In particular, the immigration of neuroectodermally differentiated cells of
[...] Read more.
Maxillofacial hard tissues have several differences compared to bones of other localizations of the human body. These could be due to the different embryological development of the jaw bones compared to the extracranial skeleton. In particular, the immigration of neuroectodermally differentiated cells of the cranial neural crest (CNC) plays an important role. These cells differ from the mesenchymal structures of the extracranial skeleton. In the ontogenesis of the jaw bones, the development via the intermediate stage of the pharyngeal arches is another special developmental feature. The aim of this review was to illustrate how the development of maxillofacial hard tissues occurs via the cranial neural crest and pharyngeal arches, and what significance this could have for relevant pathologies in maxillofacial surgery, dentistry and orthodontic therapy. The pathogenesis of various growth anomalies and certain syndromes will also be discussed.
Full article
►▼
Show Figures
Open AccessArticle
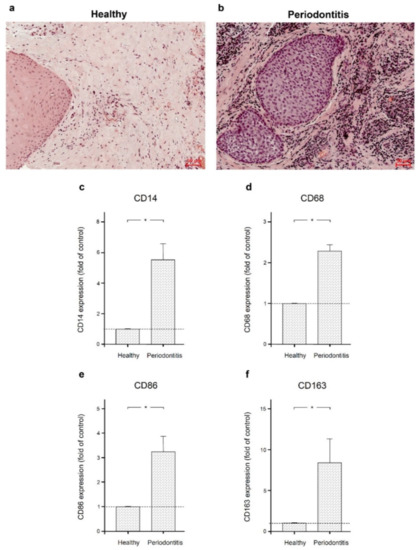
Filifactor alocis and Tumor Necrosis Factor-Alpha Stimulate Synthesis of Visfatin by Human Macrophages
by
Andressa Vilas Boas Nogueira, Marjan Nokhbehsaim, Anna Damanaki, Sigrun Eick, Christian Kirschneck, Agnes Schröder, Jonathan Jantsch and James Deschner
Cited by 11 | Viewed by 1983
Abstract
There is little known about the effect of the periodontopathogen
Filifactor alocis on macrophages as key cells of the innate immune defense in the periodontium. Therefore, the aim of the present study was to investigate the effect of
F. alocis and additionally of
[...] Read more.
There is little known about the effect of the periodontopathogen
Filifactor alocis on macrophages as key cells of the innate immune defense in the periodontium. Therefore, the aim of the present study was to investigate the effect of
F. alocis and additionally of the pro-inflammatory cytokine tumor necrosis factor-alpha (TNFα) on visfatin and other pro-inflammatory and proteolytic molecules associated with periodontitis in human macrophages. The presence of macrophage markers CD14, CD86, CD68, and CD163 was examined in gingival biopsies from healthy individuals and periodontitis patients. Human macrophages were incubated with
F. alocis and TNFα for up to 2 d. The effects of both stimulants on macrophages were determined by real-time PCR, ELISA, immunocytochemistry, and immunofluorescence.
F. alocis was able to significantly stimulate the synthesis of visfatin by human macrophages using TLR2 and MAPK pathways. In addition to visfatin,
F. alocis was also able to increase the synthesis of cyclooxygenase 2, TNFα, and matrix metalloproteinase 1. Like
F. alocis, TNFα was also able to stimulate the production of these proinflammatory and proteolytic molecules. Our results highlight the pathogenetic role of
F. alocis in periodontal diseases and also underline the involvement of visfatin in the aetiopathogenesis of periodontitis.
Full article
►▼
Show Figures
Open AccessArticle
Dietary Salt Accelerates Orthodontic Tooth Movement by Increased Osteoclast Activity
by
Agnes Schröder, Joshua Gubernator, Alexandra Leikam, Ute Nazet, Fabian Cieplik, Jonathan Jantsch, Patrick Neubert, Jens Titze, Peter Proff and Christian Kirschneck
Cited by 4 | Viewed by 2395
Abstract
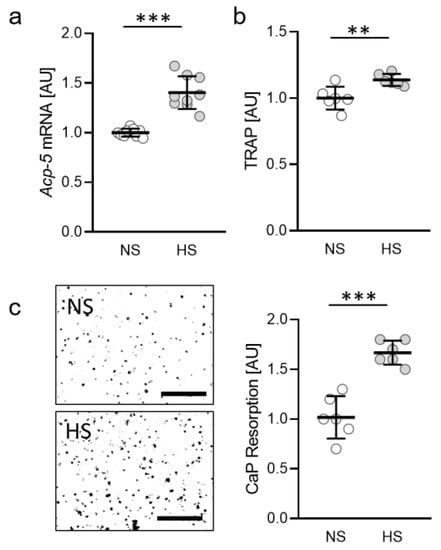
Dietary salt uptake and inflammation promote sodium accumulation in tissues, thereby modulating cells like macrophages and fibroblasts. Previous studies showed salt effects on periodontal ligament fibroblasts and on bone metabolism by expression of nuclear factor of activated T-cells-5 (NFAT-5). Here, we investigated the
[...] Read more.
Dietary salt uptake and inflammation promote sodium accumulation in tissues, thereby modulating cells like macrophages and fibroblasts. Previous studies showed salt effects on periodontal ligament fibroblasts and on bone metabolism by expression of nuclear factor of activated T-cells-5 (NFAT-5). Here, we investigated the impact of salt and NFAT-5 on osteoclast activity and orthodontic tooth movement (OTM). After treatment of osteoclasts without (NS) or with additional salt (HS), we analyzed gene expression and the release of tartrate-resistant acid phosphatase and calcium phosphate resorption. We kept wild-type mice and mice lacking NFAT-5 in myeloid cells either on a low, normal or high salt diet and inserted an elastic band between the first and second molar to induce OTM. We analyzed the expression of genes involved in bone metabolism, periodontal bone loss, OTM and bone density. Osteoclast activity was increased upon HS treatment. HS promoted periodontal bone loss and OTM and was associated with reduced bone density. Deletion of NFAT-5 led to increased osteoclast activity with NS, whereas we detected impaired OTM in mice. Dietary salt uptake seems to accelerate OTM and induce periodontal bone loss due to reduced bone density, which may be attributed to enhanced osteoclast activity. NFAT-5 influences this reaction to HS, as we detected impaired OTM and osteoclast activity upon deletion.
Full article
►▼
Show Figures
Open AccessArticle
Regulation of Anti-Apoptotic SOD2 and BIRC3 in Periodontal Cells and Tissues
by
Birgit Rath-Deschner, Andressa Vilas Boas Nogueira, Svenja Memmert, Marjan Nokhbehsaim, Joni Augusto Cirelli, Sigrun Eick, Nicolai Miosge, Christian Kirschneck, Marco Kesting, James Deschner, Andreas Jäger and Anna Damanaki
Cited by 11 | Viewed by 2024
Abstract
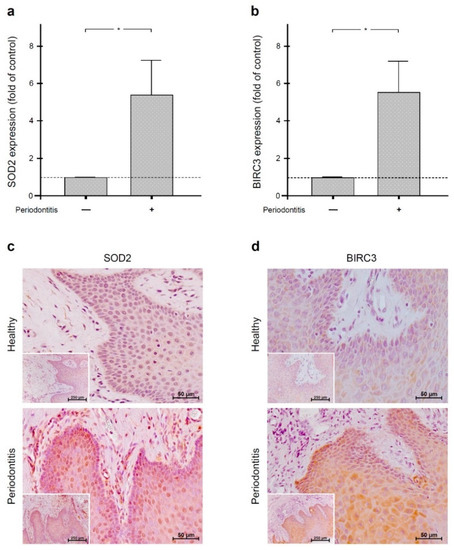
The aim of the study was to clarify whether orthodontic forces and periodontitis interact with respect to the anti-apoptotic molecules superoxide dismutase 2 (SOD2) and baculoviral IAP repeat-containing protein 3 (BIRC3). SOD2, BIRC3, and the apoptotic markers caspases 3 (CASP3) and 9 (CASP9)
[...] Read more.
The aim of the study was to clarify whether orthodontic forces and periodontitis interact with respect to the anti-apoptotic molecules superoxide dismutase 2 (SOD2) and baculoviral IAP repeat-containing protein 3 (BIRC3). SOD2, BIRC3, and the apoptotic markers caspases 3 (CASP3) and 9 (CASP9) were analyzed in gingiva from periodontally healthy and periodontitis subjects by real-time PCR and immunohistochemistry. SOD2 and BIRC3 were also studied in gingiva from rats with experimental periodontitis and/or orthodontic tooth movement. Additionally, SOD2 and BIRC3 levels were examined in human periodontal fibroblasts incubated with
Fusobacterium nucleatum and/or subjected to mechanical forces. Gingiva from periodontitis patients showed significantly higher SOD2, BIRC3, CASP3, and CASP9 levels than periodontally healthy gingiva. SOD2 and BIRC3 expressions were also significantly increased in the gingiva from rats with experimental periodontitis, but the upregulation of both molecules was significantly diminished in the concomitant presence of orthodontic tooth movement. In vitro, SOD2 and BIRC3 levels were significantly increased by
F. nucleatum, but this stimulatory effect was also significantly inhibited by mechanical forces. Our study suggests that SOD2 and BIRC3 are produced in periodontal infection as a protective mechanism against exaggerated apoptosis. In the concomitant presence of orthodontic forces, this protective anti-apoptotic mechanism may get lost.
Full article
►▼
Show Figures
Open AccessArticle
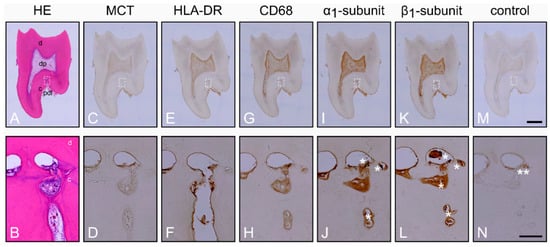
Inflammation in the Human Periodontium Induces Downregulation of the α1- and β1-Subunits of the sGC in Cementoclasts
by
Yüksel Korkmaz, Behrus Puladi, Kerstin Galler, Peer W. Kämmerer, Agnes Schröder, Lina Gölz, Tim Sparwasser, Wilhelm Bloch, Andreas Friebe and James Deschner
Cited by 3 | Viewed by 2807
Abstract
Nitric oxide (NO) binds to soluble guanylyl cyclase (sGC), activates it in a reduced oxidized heme iron state, and generates cyclic Guanosine Monophosphate (cGMP), which results in vasodilatation and inhibition of osteoclast activity. In inflammation, sGC is oxidized and becomes insensitive to NO.
[...] Read more.
Nitric oxide (NO) binds to soluble guanylyl cyclase (sGC), activates it in a reduced oxidized heme iron state, and generates cyclic Guanosine Monophosphate (cGMP), which results in vasodilatation and inhibition of osteoclast activity. In inflammation, sGC is oxidized and becomes insensitive to NO. NO- and heme-independent activation of sGC requires protein expression of the α
1- and β
1-subunits. Inflammation of the periodontium induces the resorption of cementum by cementoclasts and the resorption of the alveolar bone by osteoclasts, which can lead to tooth loss. As the presence of sGC in cementoclasts is unknown, we investigated the α
1- and β
1-subunits of sGC in cementoclasts of healthy and inflamed human periodontium using double immunostaining for CD68 and cathepsin K and compared the findings with those of osteoclasts from the same sections. In comparison to cementoclasts in the healthy periodontium, cementoclasts under inflammatory conditions showed a decreased staining intensity for both α
1- and β
1-subunits of sGC, indicating reduced protein expression of these subunits. Therefore, pharmacological activation of sGC in inflamed periodontal tissues in an NO- and heme-independent manner could be considered as a new treatment strategy to inhibit cementum resorption.
Full article
►▼
Show Figures
Open AccessReview
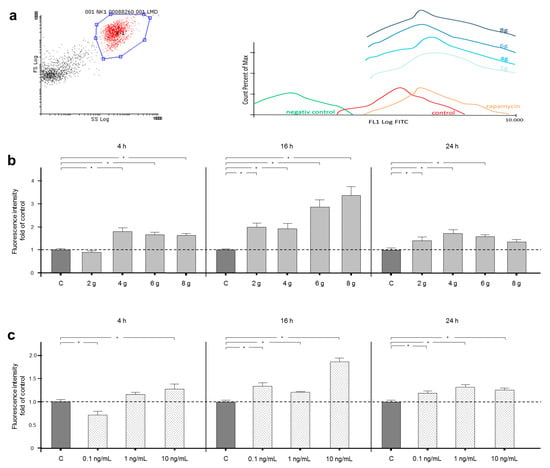
Influence of Natural Killer Cells and Natural Killer T Cells on Periodontal Disease: A Systematic Review of the Current Literature
by
Andreas Seidel, Corinna L. Seidel, Matthias Weider, Rüdiger Junker, Lina Gölz and Helga Schmetzer
Cited by 11 | Viewed by 3112
Abstract
Natural killer (NK) cells, as members of the innate immune system, and natural killer T (NKT) cells, bridging innate and adaptive immunity, play a prominent role in chronic inflammatory diseases and cancerogenesis, yet have scarcely been examined in oral diseases. Therefore, systematic research
[...] Read more.
Natural killer (NK) cells, as members of the innate immune system, and natural killer T (NKT) cells, bridging innate and adaptive immunity, play a prominent role in chronic inflammatory diseases and cancerogenesis, yet have scarcely been examined in oral diseases. Therefore, systematic research on the latest literature focusing on NK/NKT cell-mediated mechanisms in periodontal disease, including the time period 1988–2020, was carried out in MEDLINE (PubMed) using a predetermined search strategy, with a final selection of 25 studies. The results showed that NK cells tend to have rather proinflammatory influences via cytokine production, cytotoxic effects, dendritic-cell-crosstalk, and autoimmune reactions, while contrarily, NKT cell-mediated mechanisms were proinflammatory and immunoregulatory, ranging from protective effects via B-cell-regulation, specific antibody production, and the suppression of autoimmunity to destructive effects via cytokine production, dendritic-cell-crosstalk, and T-/B-cell interactions. Since NK cells seem to have a proinflammatory role in periodontitis, further research should focus on the proinflammatory and immunoregulatory properties of NKT cells in order to create, in addition to antibacterial strategies in dental inflammatory disease, novel anti-inflammatory therapeutic approaches modulating host immunity towards dental health.
Full article
►▼
Show Figures
Open AccessArticle
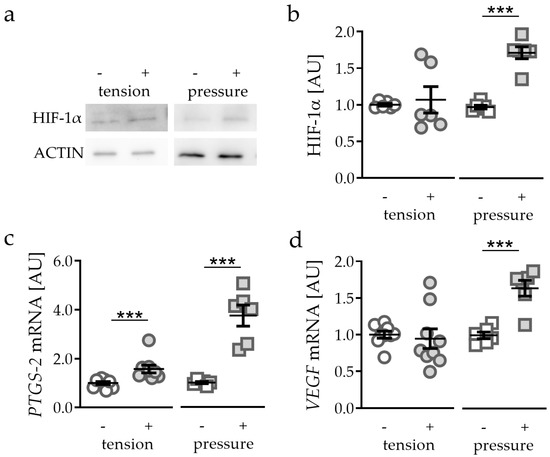
Role and Regulation of Mechanotransductive HIF-1α Stabilisation in Periodontal Ligament Fibroblasts
by
Christian Kirschneck, Magdalena Thuy, Alexandra Leikam, Svenja Memmert, James Deschner, Anna Damanaki, Gerrit Spanier, Peter Proff, Jonathan Jantsch and Agnes Schröder
Cited by 8 | Viewed by 2275
Abstract
Orthodontic tooth movement (OTM) creates compressive and tensile strain in the periodontal ligament, causing circulation disorders. Hypoxia-inducible factor 1α (HIF-1α) has been shown to be primarily stabilised by compression, but not hypoxia in periodontal ligament fibroblasts (PDLF) during mechanical strain, which are key
[...] Read more.
Orthodontic tooth movement (OTM) creates compressive and tensile strain in the periodontal ligament, causing circulation disorders. Hypoxia-inducible factor 1α (HIF-1α) has been shown to be primarily stabilised by compression, but not hypoxia in periodontal ligament fibroblasts (PDLF) during mechanical strain, which are key regulators of OTM. This study aimed to elucidate the role of heparan sulfate integrin interaction and downstream kinase phosphorylation for HIF-1α stabilisation under compressive and tensile strain and to which extent downstream synthesis of VEGF and prostaglandins is HIF-1α-dependent in a model of simulated OTM in PDLF. PDLF were subjected to compressive or tensile strain for 48 h. In various setups HIF-1α was experimentally stabilised (DMOG) or destabilised (YC-1) and mechanotransduction was inhibited by surfen and genistein. We found that HIF-1α was not stabilised by tensile, but rather by compressive strain. HIF-1α stabilisation had an inductive effect on prostaglandin and VEGF synthesis. As expected, HIF-1α destabilisation reduced VEGF expression, whereas prostaglandin synthesis was increased. Inhibition of integrin mechanotransduction via surfen or genistein prevented stabilisation of HIF-1α. A decrease in VEGF expression was observed, but not in prostaglandin synthesis. Stabilisation of HIF-1α via integrin mechanotransduction and downstream phosphorylation of kinases seems to be essential for the induction of VEGF, but not prostaglandin synthesis by PDLF during compressive (but not tensile) orthodontic strain.
Full article
►▼
Show Figures
Open AccessArticle
Regulation of Autophagic Signaling by Mechanical Loading and Inflammation in Human PDL Fibroblasts
by
Kim Blawat, Alexandra Mayr, Miriam Hardt, Christian Kirschneck, Marjan Nokhbehsaim, Christian Behl, James Deschner, Andreas Jäger and Svenja Memmert
Cited by 13 | Viewed by 1824
Abstract
Autophagy (cellular self-consumption) is a crucial adaptation mechanism during cellular stress conditions. This study aimed to examine how this important process is regulated in human periodontal ligament (PDL) fibroblasts by mechanical and inflammatory stress conditions and whether the mammalian target of rapamycin (mTOR)
[...] Read more.
Autophagy (cellular self-consumption) is a crucial adaptation mechanism during cellular stress conditions. This study aimed to examine how this important process is regulated in human periodontal ligament (PDL) fibroblasts by mechanical and inflammatory stress conditions and whether the mammalian target of rapamycin (mTOR) signaling pathway is involved. Autophagy was quantified by flow cytometry. Qualitative protein phosphorylation profiling of the mTOR pathway was carried out. Effects of mTOR regulation were assessed by quantification of important synthesis product collagen 1, cell proliferation and cell death with real-time PCR and flow cytometry. Autophagy as a response to mechanical or inflammatory treatment in PDL fibroblasts was dose and time dependent. In general, autophagy was induced by stress stimulation. Phosphorylation analysis of mTOR showed regulatory influences of mechanical and inflammatory stimulation on crucial target proteins. Regulation of mTOR was also detectable via changes in protein synthesis and cell proliferation. Physiological pressure had cell-protective effects (
p = 0.025), whereas overload increased cell death (
p = 0.003), which was also promoted in long-term inflammatory treatment (
p < 0.001). Our data provide novel insights about autophagy regulation by mechanical and inflammatory stress conditions in human PDL fibroblasts. Our results suggest some involvement of the mTOR pathway in autophagy and cell fate regulation under the named conditions.
Full article
►▼
Show Figures
Open AccessArticle
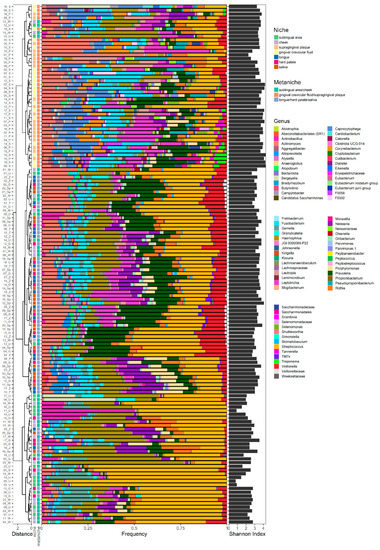
Defining Metaniches in the Oral Cavity According to Their Microbial Composition and Cytokine Profile
by
Corinna L. Seidel, Roman G. Gerlach, Patrick Wiedemann, Matthias Weider, Gabriele Rodrian, Michael Hader, Benjamin Frey, Udo S. Gaipl, Aline Bozec, Fabian Cieplik, Christian Kirschneck, Christian Bogdan and Lina Gölz
Cited by 16 | Viewed by 2635
Abstract
The human oral microbiota consists of over 700 widespread taxa colonizing the oral cavity in several anatomically diverse oral niches. Lately, sequencing of the 16S rRNA genes has become an acknowledged, culture-independent method to characterize the oral microbiota. However, only a small amount
[...] Read more.
The human oral microbiota consists of over 700 widespread taxa colonizing the oral cavity in several anatomically diverse oral niches. Lately, sequencing of the 16S rRNA genes has become an acknowledged, culture-independent method to characterize the oral microbiota. However, only a small amount of data are available concerning microbial differences between oral niches in periodontal health and disease. In the context of periodontitis, the cytokine expression in the gingival crevicular fluid has been studied in detail, whereas little is known about the cytokine profile in hard and soft tissue biofilms. In order to characterize oral niches in periodontal health, the oral microbiota and cytokine pattern were analyzed at seven different sites (plaque (P), gingival crevicular fluid (GCF), saliva (S), tongue (T), hard palate (HP), cheek (C) and sublingual area (U)) of 20 young adults using next-generation sequencing and multiplex immunoassays. Site-specific microbial compositions were detected, which clustered into three distinct metaniches (“P-GCF”, “S-T-HP” and “C-U”) and were associated with niche-/metaniche-specific cytokine profiles. Our findings allow the definition of distinct metaniches according to their microbial composition, partly reflected by their cytokine profile, and provide new insights into microenvironmental similarities between anatomical diverse oral niches.
Full article
►▼
Show Figures
Open AccessArticle
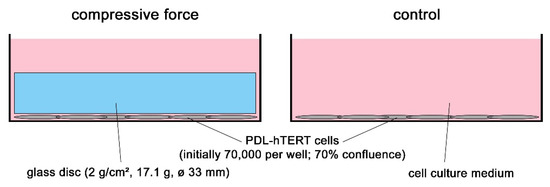
A Human Periodontal Ligament Fibroblast Cell Line as a New Model to Study Periodontal Stress
by
Matthias Weider, Agnes Schröder, Denitsa Docheva, Gabriele Rodrian, Isabel Enderle, Corinna Lesley Seidel, Darja Andreev, Michael Wegner, Aline Bozec, James Deschner, Christian Kirschneck, Peter Proff and Lina Gölz
Cited by 12 | Viewed by 2529
Abstract
The periodontal ligament (PDL) is exposed to different kinds of mechanical stresses such as bite force or orthodontic tooth movement. A simple and efficient model to study molecular responses to mechanical stress is the application of compressive force onto primary human periodontal ligament
[...] Read more.
The periodontal ligament (PDL) is exposed to different kinds of mechanical stresses such as bite force or orthodontic tooth movement. A simple and efficient model to study molecular responses to mechanical stress is the application of compressive force onto primary human periodontal ligament fibroblasts via glass disks. Yet, this model suffers from the need for primary cells from human donors which have a limited proliferative capacity. Here we show that an immortalized cell line, PDL-hTERT, derived from primary human periodontal ligament fibroblasts exhibits characteristic responses to glass disk-mediated compressive force resembling those of primary cells. These responses include induction and secretion of pro-inflammatory markers, changes in expression of extracellular matrix-reorganizing genes and induction of genes related to angiogenesis, osteoblastogenesis and osteoclastogenesis. The fact that PDL-hTERT cells can easily be transfected broadens their usefulness, as molecular gain- and loss-of-function studies become feasible.
Full article
►▼
Show Figures
Open AccessArticle
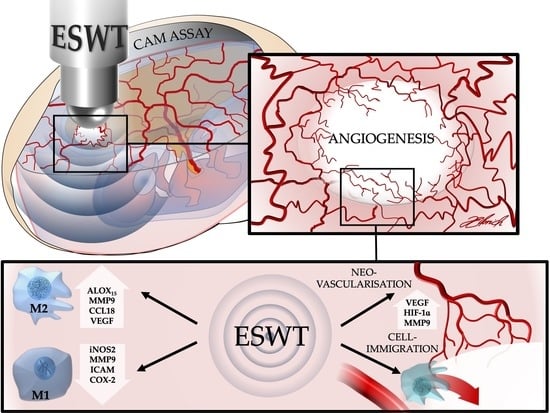
In Vivo Modulation of Angiogenesis and Immune Response on a Collagen Matrix via Extracorporeal Shockwaves
by
Diana Heimes, Nadine Wiesmann, Jonas Eckrich, Juergen Brieger, Stefan Mattyasovszky, Peter Proff, Manuel Weber, James Deschner, Bilal Al-Nawas and Peer W. Kämmerer
Cited by 20 | Viewed by 2838
Abstract
The effective management of tissue integration and immunological responses to transplants decisively co-determines the success of soft and hard tissue reconstruction. The aim of this in vivo study was to evaluate the eligibility of extracorporeal shock wave therapy (ESWT) with respect to its
[...] Read more.
The effective management of tissue integration and immunological responses to transplants decisively co-determines the success of soft and hard tissue reconstruction. The aim of this in vivo study was to evaluate the eligibility of extracorporeal shock wave therapy (ESWT) with respect to its ability to modulate angiogenesis and immune response to a collagen matrix (CM) for tissue engineering in the chorioallantoic membrane (CAM) assay, which is performed with fertilized chicken eggs. CM were placed on the CAM on embryonic development day (EDD) 7; at EDD-10, ESWT was conducted at 0.12 mJ/mm
2 with 500 impulses each. One and four days later, angiogenesis represented by vascularized area, vessel density, and vessel junctions as well as HIF-1α and VEGF gene expression were evaluated. Furthermore, immune response (iNOS2, MMP-9, and MMP-13 via qPCR) was assessed and compared between ESWT- and non-ESWT-groups. At EDD-14, the vascularized area (+115% vs. +26%) and the increase in vessel junctions (+751% vs. +363%) were significantly higher in the ESWT-group. ESWT significantly increased MMP-9 gene expression at EDD-11 and significantly decreased MMP-13 gene expression at EDD-14 as compared to the controls. Using the CAM assay, an enhanced angiogenesis and neovascularization in CM after ESWT were observed. Furthermore, ESWT could reduce the inflammatory activity after a latency of four days.
Full article
►▼
Show Figures