State-of-the-Art Molecular Endocrinology and Metabolism in Spain
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
This topical collection aims to provide an up-to-date and comprehensive view on the state-of-the-art of endocrinology and metabolism in Spain.
Based on long-term traditions of fundamental science in Spain, we would like to provide a comprehensive insight into the state-of-the-art of research activities in the country. All kinds of articles are welcome, including contributions about original investigations, as well as reviews in the field. The covered topics of interest include, but are not limited to, the following:
- Endocrine systems and endocrine-related diseases;
- Molecular, cellular, genetic, epigenetic, developmental approaches, and animal models;
- Novel insights into physiology, pathophysiology, and therapeutics;
- Neuroendocrinology and neuroendocrine control of endocrine axes;
- Classical glands (thyroid, adrenal, pituitary, parathyroid, testis, ovary, pituitary, etc.) and other endocrine systems: gut, bone, liver, etc.;
- Lipids and bone metabolism;
- Hormones, paracrine factors, receptors and binding components, nuclear receptors membrane receptors, signal transduction pathway;
- Steroid biosynthetic enzymes, metabolism of hormones, neurotransmitters, etc.;
- Cellular interactions and factors involved;
- Energy expenditure;
- Diabetes;
- Infertility and reproductive diseases;
- Obesity;
- Osteoporosis;
- Aging;
- Endocrine-related tumor and cancer;
- Endocrine disruption;
- Cross-disciplinary and integrative studies;
- Comparative aspects of endocrinology.
Dr. Carolina Serena
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
There is an Article Processing Charge (APC) for publication in this
open access journal. For details about the APC please see here.
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (9 papers)
2023
Open AccessArticle
Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation?
by
Bernat Soria, Natalia Escacena, Aitor Gonzaga, Barbara Soria-Juan, Etelvina Andreu, Abdelkrim Hmadcha, Ana Maria Gutierrez-Vilchez, Gladys Cahuana, Juan R. Tejedo, Antonio De la Cuesta, Manuel Miralles, Susana García-Gómez and Luis Hernández-Blasco
Viewed by 1896
Abstract
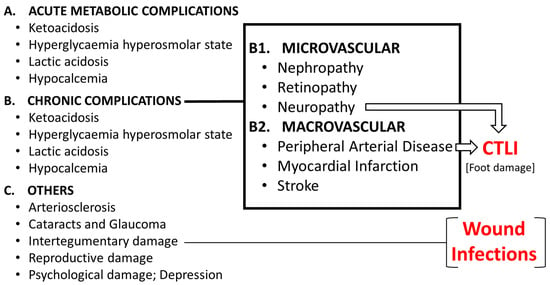
Globally, a leg is amputated approximately every 30 seconds, with an estimated 85 percent of these amputations being attributed to complications arising from diabetic foot ulcers (DFU), as stated by the American Diabetes Association. Peripheral arterial disease (PAD) is a risk factor resulting
[...] Read more.
Globally, a leg is amputated approximately every 30 seconds, with an estimated 85 percent of these amputations being attributed to complications arising from diabetic foot ulcers (DFU), as stated by the American Diabetes Association. Peripheral arterial disease (PAD) is a risk factor resulting in DFU and can, either independently or in conjunction with diabetes, lead to recurring, slow-healing ulcers and amputations. According to guidelines amputation is the recommended treatment for patients with no-option critical ischemia of the limb (CTLI). In this article we propose cell therapy as an alternative strategy for those patients. We also suggest the optimal time-frame for an effective therapy, such as implanting autologous mononuclear cells (MNCs), autologous and allogeneic mesenchymal stromal cells (MSC) as these treatments induce neuropathy relief, regeneration of the blood vessels and tissues, with accelerated ulcer healing, with no serious side effects, proving that advanced therapy medicinal product (ATMPs) application is safe and effective and, hence, can significantly prevent limb amputation.
Full article
►▼
Show Figures
Open AccessArticle
Correlational Study of Aminopeptidase Activities between Left or Right Frontal Cortex versus the Hypothalamus, Pituitary, Adrenal Axis of Spontaneously Hypertensive Rats Treated with Hypotensive or Hypertensive Agents
by
Isabel Prieto, Ana Belén Segarra, Inmaculada Banegas, Magdalena Martínez-Cañamero, Raquel Durán, Francisco Vives, Germán Domínguez-Vías and Manuel Ramírez-Sánchez
Viewed by 743
Abstract
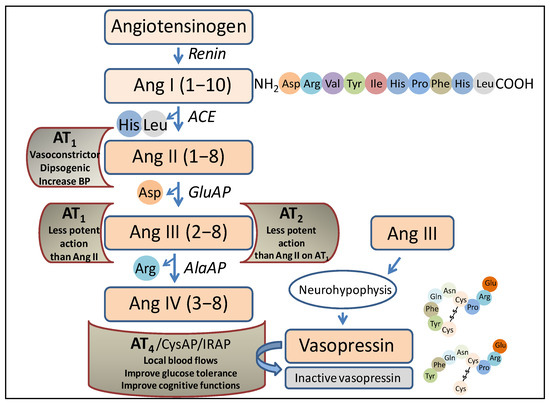
It has been suggested that the neuro-visceral integration works asymmetrically and that this asymmetry is dynamic and modifiable by physio-pathological influences. Aminopeptidases of the renin–angiotensin system (angiotensinases) have been shown to be modifiable under such conditions. This article analyzes the interactions of these
[...] Read more.
It has been suggested that the neuro-visceral integration works asymmetrically and that this asymmetry is dynamic and modifiable by physio-pathological influences. Aminopeptidases of the renin–angiotensin system (angiotensinases) have been shown to be modifiable under such conditions. This article analyzes the interactions of these angiotensinases between the left or right frontal cortex (FC) and the same enzymes in the hypothalamus (HT), pituitary (PT), adrenal (AD) axis (HPA) in control spontaneously hypertensive rats (SHR), in SHR treated with a hypotensive agent in the form of captopril (an angiotensin-converting enzyme inhibitor), and in SHR treated with a hypertensive agent in the form of the L-Arginine hypertensive analogue L-NG-Nitroarginine Methyl Ester (L-NAME). In the control SHR, there were significant negative correlations between the right FC with HPA and positive correlations between the left FC and HPA. In the captopril group, the predominance of negative correlations between the right FC and HPA and positive correlations between the HPA and left FC was maintained. In the L-NAME group, a radical change in all types of interactions was observed; particularly, there was an inversion in the predominance of negative correlations between the HPA and left FC. These results indicated a better balance of neuro-visceral interactions after captopril treatment and an increase in these interactions in the hypertensive animals, especially in those treated with L-NAME.
Full article
►▼
Show Figures
Open AccessArticle
GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats
by
Alba Miguéns-Gómez, Marta Sierra-Cruz, M. Teresa Blay, Esther Rodríguez-Gallego, Raúl Beltrán-Debón, Ximena Terra, Montserrat Pinent and Anna Ardévol
Viewed by 1027
Abstract
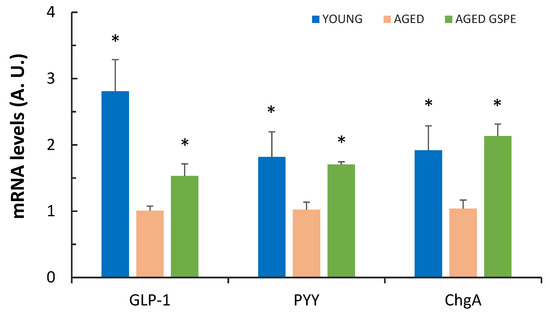
The impact that healthy aging can have on society has raised great interest in understanding aging mechanisms. However, the effects this biological process may have on the gastrointestinal tract (GIT) have not yet been fully described. Results in relation to changes observed in
[...] Read more.
The impact that healthy aging can have on society has raised great interest in understanding aging mechanisms. However, the effects this biological process may have on the gastrointestinal tract (GIT) have not yet been fully described. Results in relation to changes observed in the enteroendocrine system along the GIT are controversial. Grape seed proanthocyanidin extracts (GSPE) have been shown to protect against several pathologies associated with aging. Based on previous results, we hypothesized that a GSPE pre-treatment could prevent the aging processes that affect the enteroendocrine system. To test this hypothesis, we treated 21-month-old female rats with GSPE for 10 days. Eleven weeks after the treatment, we analyzed the effects of GSPE by comparing these aged animals with young animals. Aging induced a greater endocrine response to stimulation in the upper GIT segments (cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1)), a decrease in the mRNA abundance of GLP-1, peptide YY (PYY) and chromogranin A (ChgA) in the colon, and an increase in colonic butyrate. GSPE-treated rats were protected against a decrease in enterohormone expression in the colon. This effect is not directly related to the abundance of microbiome or short-chain fatty acids (SCFA) at this location. GSPE may therefore be effective in preventing a decrease in the colonic abundance of enterohormone expression induced by aging.
Full article
►▼
Show Figures
Open AccessArticle
Metabolic Overlap between Alzheimer’s Disease and Metabolic Syndrome Identifies the PVRL2 Gene as a New Modulator of Diabetic Dyslipidemia
by
Montse Guardiola, Gerard Muntané, Iris Martínez, Lourdes Martorell, Josefa Girona, Daiana Ibarretxe, Núria Plana, María J. Bullido, Elisabet Vilella and Josep Ribalta
Cited by 2 | Viewed by 1454
Abstract
Background: Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM) share metabolic alterations such as abnormal insulin and lipid metabolism and have some common genetic factors such as
APOE genotype. Taking this into account, we hypothesized that we could identify common genetic factors
[...] Read more.
Background: Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM) share metabolic alterations such as abnormal insulin and lipid metabolism and have some common genetic factors such as
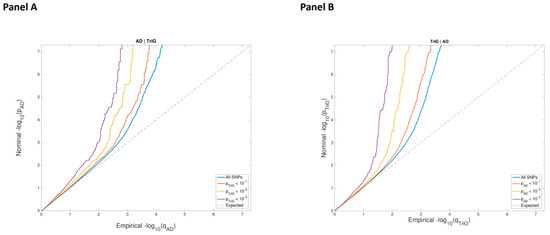
APOE genotype. Taking this into account, we hypothesized that we could identify common genetic factors involved in the development of diabetes and cardiovascular diseases. Methodology: We first genotyped 48 single nucleotide polymorphisms (SNPs) previously associated with AD in a cohort composed of 330 patients with cognitive impairment (CI) to assess their association with plasma lipids. Second, we conducted pleiotropy-informed conjunctional false discovery rate (FDR) analysis designed to identify shared variants between AD and plasma lipid levels. Finally, we used the SNPs to be found associated with lipid parameters and AD to search for associations with lipoprotein parameters in 281 patients with cardiometabolic risk. Results: Five SNPs were significantly associated with lower levels of cholesterol transported in remnant lipoprotein particles (RLPc) in subjects with CI; among these SNPs was the rs73572039 variant in
PVRL2. Stratified QQ-plots were conducted on GWAS designed for AD and triglycerides (TG). The cross-trait analysis resulted in a total of 22 independent genomic loci associated with both AD and TG levels with a conjFDR < 0.05. Among these loci, two pleiotropic variants were located in
PVRL2 (rs12978931 and rs11667640). The three SNPs in
PVRL2 were significantly associated with RLPc, TG, and number of circulating VLDL and HDL particles in subjects with cardiometabolic risk. Conclusions: We have identified three variants in
PVRL2 that predispose individuals to AD that also influence the lipid profile that confers cardiovascular risk in T2DM subjects.
PVRL2 is a potential new modulating factor of atherogenic dyslipidemia.
Full article
►▼
Show Figures
Open AccessArticle
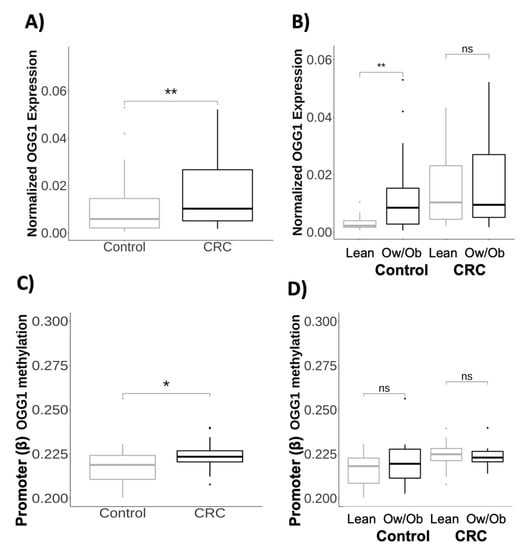
8-Oxoguanine DNA Glycosylase 1 Upregulation as a Risk Factor for Obesity and Colorectal Cancer
by
Jesús Pilo, Libia Alejandra García-Flores, Mercedes Clemente-Postigo, Isabel Arranz-Salas, Julia Alcaide, Maria Ramos-Fernandez, José Lozano, Hatim Boughanem, Pallavi Kompella and Manuel Macías-González
Viewed by 1798
Abstract
DNA damage has been extensively studied as a potentially helpful tool in assessing and preventing cancer, having been widely associated with the deregulation of DNA damage repair (DDR) genes and with an increased risk of cancer. Adipose tissue and tumoral cells engage in
[...] Read more.
DNA damage has been extensively studied as a potentially helpful tool in assessing and preventing cancer, having been widely associated with the deregulation of DNA damage repair (DDR) genes and with an increased risk of cancer. Adipose tissue and tumoral cells engage in a reciprocal interaction to establish an inflammatory microenvironment that enhances cancer growth by modifying epigenetic and gene expression patterns. Here, we hypothesize that 8-oxoguanine DNA glycosylase 1 (OGG1)—a DNA repair enzyme—may represent an attractive target that connects colorectal cancer (CRC) and obesity. In order to understand the mechanisms underlying the development of CRC and obesity, the expression and methylation of DDR genes were analyzed in visceral adipose tissue from CRC and healthy participants. Gene expression analysis revealed an upregulation of
OGG1 expression in CRC participants (
p < 0.005) and a downregulation of
OGG1 in normal-weight healthy patients (
p < 0.05). Interestingly, the methylation analysis showed the hypermethylation of
OGG1 in CRC patients (
p < 0.05). Moreover, expression patterns of
OGG1 were found to be regulated by vitamin D and inflammatory genes. In general, our results showed evidence that
OGG1 can regulate CRC risk through obesity and may act as a biomarker for CRC.
Full article
►▼
Show Figures
Open AccessArticle
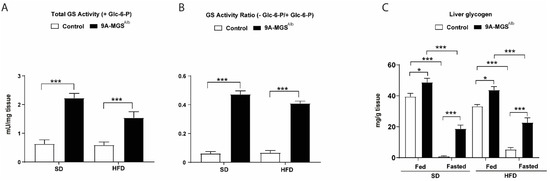
Active Glycogen Synthase in the Liver Prevents High-Fat Diet-Induced Glucose Intolerance, Decreases Food Intake, and Lowers Body Weight
by
Iliana López-Soldado, Joan J. Guinovart and Jordi Duran
Cited by 1 | Viewed by 1545
Abstract
Many lines of evidence demonstrate a correlation between liver glycogen content and food intake. We previously demonstrated that mice overexpressing protein targeting to glycogen (PTG) specifically in the liver—which have increased glycogen content in this organ—are protected from high-fat diet (HFD)-induced obesity by
[...] Read more.
Many lines of evidence demonstrate a correlation between liver glycogen content and food intake. We previously demonstrated that mice overexpressing protein targeting to glycogen (PTG) specifically in the liver—which have increased glycogen content in this organ—are protected from high-fat diet (HFD)-induced obesity by reduced food intake. However, the use of PTG to increase liver glycogen implies certain limitations. PTG stimulates glycogen synthesis but also inhibits the enzyme responsible for glycogen degradation. Furthermore, as PTG is a regulatory subunit of protein phosphatase 1 (PP1), which regulates many cellular functions, its overexpression could have side effects beyond the regulation of glycogen metabolism. Therefore, it is necessary to determine whether the direct activation of glycogen synthesis, without affecting its degradation or other cellular functions, has the same effects. To this end, we generated mice overexpressing a non-inactivatable form of glycogen synthase (GS) specifically in the liver (9A-MGS
Alb mice). Control and 9a-MGS
Alb mice were fed a standard diet (SD) or HFD for 16 weeks. Glucose tolerance and feeding behavior were analyzed. 9A-MGS
Alb mice showed an increase in hepatic glycogen in fed and fasting conditions. When fed an HFD, these animals preserved their hepatic energy state, had a reduced food intake, and presented a lower body weight and fat mass than control animals, without changes in energy expenditure. Furthermore, 9A-MGS
Alb animals showed improved glucose tolerance when fed an SD or HFD. Moreover, liver triacylglycerol levels that were increased after HFD feeding were lower in these mice. These results confirm that increased liver glycogen stores contribute to decreased appetite and improve glucose tolerance in mice fed an HFD. On the basis of our findings, strategies to preserve hepatic glycogen stores emerge as potential treatments for obesity and hyperglycemia.
Full article
►▼
Show Figures
Open AccessArticle
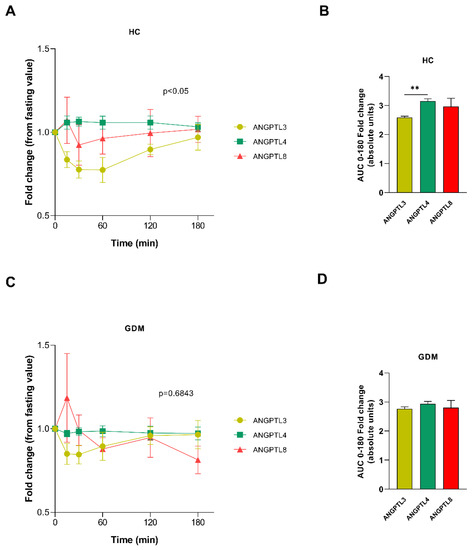
The ANGPTL3-4-8 Axis in Normal Gestation and in Gestational Diabetes, and Its Potential Involvement in Fetal Growth
by
Sergiy Klid, Elsa Maymó-Masip, Francisco Algaba-Chueca, Mónica Ballesteros, Montserrat Inglès-Puig, Albert Guarque, Ana Madeira, Carlos Jareño, Joan Vendrell, Sonia Fernández-Veledo and Ana Megía
Cited by 3 | Viewed by 1489
Abstract
Dyslipidemia in gestational diabetes has been associated with worse perinatal outcomes. The ANGPTL3-4-8 axis regulates lipid metabolism, especially in the transition from fasting to feeding. In this study, we evaluated the response of ANGPTL3, 4, and 8 after the intake of a mixed
[...] Read more.
Dyslipidemia in gestational diabetes has been associated with worse perinatal outcomes. The ANGPTL3-4-8 axis regulates lipid metabolism, especially in the transition from fasting to feeding. In this study, we evaluated the response of ANGPTL3, 4, and 8 after the intake of a mixed meal in women with normal glucose tolerance and gestational diabetes, and we assessed their gene expressions in different placental locations. Regarding the circulating levels of ANGPTL3, 4, and 8, we observed an absence of ANGPTL4 response after the intake of the meal in the GDM group compared to its presence in the control group. At the placental level, we observed a glucose tolerance-dependent expression pattern of
ANGPTL3 between the two placental sides. When we compared the GDM pregnancies with the control pregnancies, a downregulation of the maternal side
ANGPTL3 expression was observed. This suggests a dysregulation of the ANGPTL3-4-8 axis in GDM, both at the circulating level after ingestion and at the level of placental expression. Furthermore, we discerned that the expressions of
ANGPTL3,
4, and
8 were related to birth weight and placental weight in the GDM group, but not in the control group, which suggests that they may play a role in regulating the transplacental passage of nutrients.
Full article
►▼
Show Figures
Open AccessReview
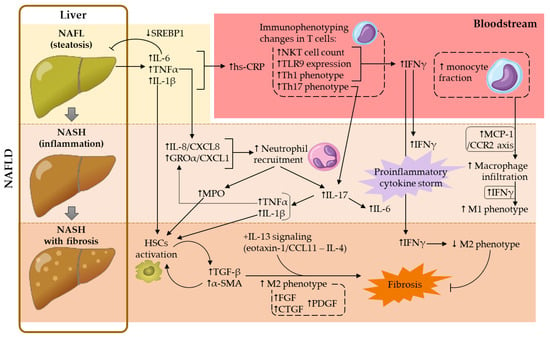
Overview of Cellular and Soluble Mediators in Systemic Inflammation Associated with Non-Alcoholic Fatty Liver Disease
by
Patrice Marques, Vera Francisco, Laura Martínez-Arenas, Ângela Carvalho-Gomes, Elena Domingo, Laura Piqueras, Marina Berenguer and Maria-Jesus Sanz
Cited by 7 | Viewed by 2264
Abstract
Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent chronic liver disease in Western countries, affecting approximately 25% of the adult population. This condition encompasses a spectrum of liver diseases characterized by abnormal accumulation of fat in liver tissue (non-alcoholic fatty liver,
[...] Read more.
Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent chronic liver disease in Western countries, affecting approximately 25% of the adult population. This condition encompasses a spectrum of liver diseases characterized by abnormal accumulation of fat in liver tissue (non-alcoholic fatty liver, NAFL) that can progress to non-alcoholic steatohepatitis (NASH), characterized by the presence of liver inflammation and damage. The latter form often coexists with liver fibrosis which, in turn, may progress to a state of cirrhosis and, potentially, hepatocarcinoma, both irreversible processes that often lead to the patient’s death and/or the need for liver transplantation. Along with the high associated economic burden, the high mortality rate among NAFLD patients raises interest, not only in the search for novel therapeutic approaches, but also in early diagnosis and prevention to reduce the incidence of NAFLD-related complications. In this line, an exhaustive characterization of the immune status of patients with NAFLD is mandatory. Herein, we attempted to gather and compare the current and relevant scientific evidence on this matter, mainly on human reports. We addressed the current knowledge related to circulating cellular and soluble mediators, particularly platelets, different leukocyte subsets and relevant inflammatory soluble mediators.
Full article
►▼
Show Figures
Open AccessReview
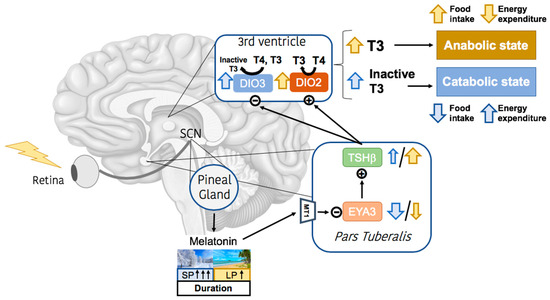
Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals
by
Èlia Navarro-Masip, Alexandre Caron, Miquel Mulero, Lluís Arola and Gerard Aragonès
Cited by 3 | Viewed by 1584
Abstract
Energy homeostasis and metabolism in mammals are strongly influenced by seasonal changes. Variations in photoperiod patterns drive adaptations in body weight and adiposity, reflecting changes in the regulation of food intake and energy expenditure. Humans also show distinct patterns of energy balance depending
[...] Read more.
Energy homeostasis and metabolism in mammals are strongly influenced by seasonal changes. Variations in photoperiod patterns drive adaptations in body weight and adiposity, reflecting changes in the regulation of food intake and energy expenditure. Humans also show distinct patterns of energy balance depending on the season, being more susceptible to gaining weight during a specific time of the year. Changes in body weight are mainly reflected by the adipose tissue, which is a key metabolic tissue and is highly affected by circannual rhythms. Mostly, in summer-like (long-active) photoperiod, adipocytes adopt a rather anabolic profile, more predisposed to store energy, while food intake increases and energy expenditure is reduced. These metabolic adaptations involve molecular modifications, some of which have been studied during the last years and are summarized in this review. In addition, there is a bidirectional relation between obesity and the seasonal responses, with obesity disrupting some of the seasonal responses observed in healthy mammals, and altered seasonality being highly associated with increased risk of developing obesity. This suggests that changes in photoperiod produce important metabolic alterations in healthy organisms. Biological rhythms impact the regulation of metabolism to different extents, some of which are already known, but further research is needed to fully understand the relationship between energy balance and seasonality.
Full article
►▼
Show Figures