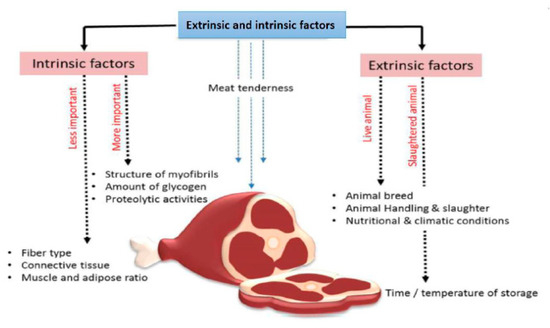
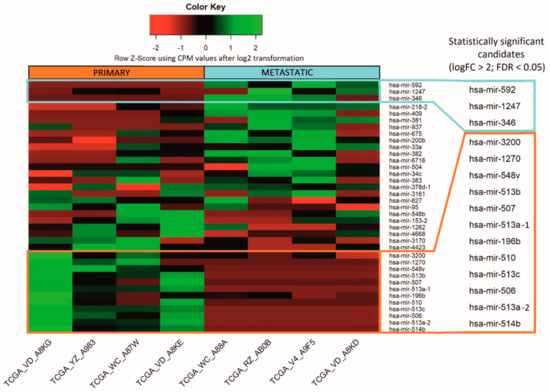
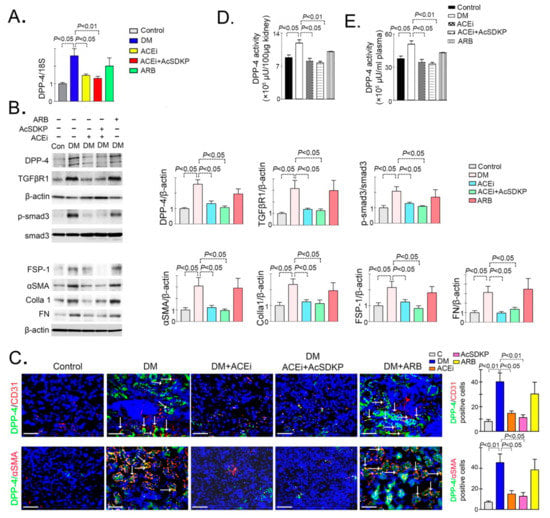
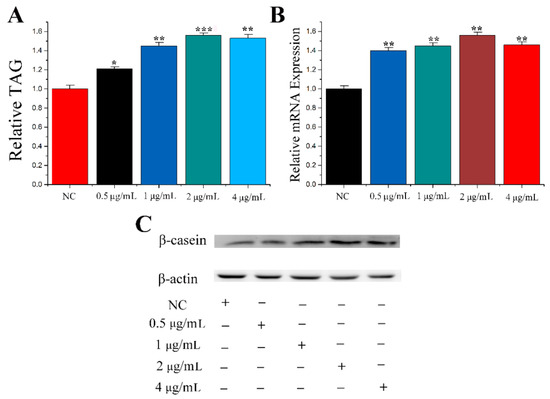
microRNA Omnibus
A topical collection in Genes (ISSN 2073-4425). This collection belongs to the section "Molecular Genetics and Genomics".
Viewed by 23712Editors
Interests: receptor-mediated delivery; liver regeneration; microRNA biogenesis; genome editing
Special Issues, Collections and Topics in MDPI journals
Interests: colorectal cancer; tumor immunology; T cells; immune cells; microbiome
Special Issues, Collections and Topics in MDPI journals
Interests: macrophages; microRNAs; epigenetics; transcription; cancer immunology; asthma; inflammatory bowel disease
Topical Collection Information
Dear Colleagues,
Since the discovery of microRNAs (miRNAs) in the early 90s, this ~ 22-nucleotide long RNA molecule has been placed in numerous biological contexts, where it conducts post-transcriptional, and perhaps even transcriptional regulation of gene expression and RNA silencing. miRNAs are present in mammals, plants and some viruses, and their function is based on the resemblance that they share with mRNAs, which allows them to bind those molecules and modulate their expression through different mechanisms such as elongation inhibition, cap initiation inhibition and mRNA decay.
The number of publications including miRNAs has grown exponentially since their first definition. Most notably, many findings have established that miRNAs are conserved through evolution, regulate the expression of hundreds of genes involved in cancer or other diseases and influence the epigenome. It is estimated that the human genome may encode over 2,000 miRNAs, and as more research is done on this topic, we keep finding novel circumstances where miRNAs play a significant role, such as in obesity, homeostasis, alcoholism and kidney disease, to name a few.
The increasing interest in miRNAs has encouraged us to prepare this topical collection, where we intend to publish new findings on miRNA research and create a collection that tracks the different progresses made in the field over the years. We invite interested authors to submit original research articles, reviews, concept papers and commentaries to this topical collection.
Prof. Clifford J. Steer
Dr. Subree Subramanian
Dr. Tilman Sanchez-Elsner
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Genes is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- microRNAs
- gene expression
- RNA silencing
- post-transcriptional regulation
- epigenetics