Feature Papers: Neurogenomics
A topical collection in Genes (ISSN 2073-4425). This collection belongs to the section "Neurogenomics".
Viewed by 10177
Share This Topical Collection
Editor
 Prof. Dr. Diego Centonze
Prof. Dr. Diego Centonze
 Prof. Dr. Diego Centonze
Prof. Dr. Diego Centonze
E-Mail
Website
Collection Editor
Department of Systems Medicine, Tor Vergata University of Rome, Via Montpellier 1, 00133 Rome, Italy
Interests: cellular neuroscience; neurodegeneration; neurogenetics
Topical Collection Information
Dear Colleagues,
This Topical Collection, “Feature Papers in Neurogenetics and Neurogenomics”, aims to collect high-quality research articles, review articles, and communications on advances in the research area of Neurogenetics and Neurogenomics. Since the aim of this issue is to illustrate, through selected works, frontier research in the field of neurogenetics and neurogenomics, we encourage Editorial Board Members of the Section “Neurogenetics and Neurogenomics” to contribute feature papers reflecting the latest progress in their research field, or to invite relevant senior experts and colleagues to make contributions to this Topical Collection. We aim to represent our Section as an attractive open-access publishing platform for neurogenetics and neurogenomics research.
Prof. Dr. Diego Centonze
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Genes is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- neurodegenerative diseases
- psychiatry
- genetics
- neurology
Published Papers (5 papers)
Open AccessArticle
Synthetic Adrenocorticotropic Peptides Modulate the Expression Pattern of Immune Genes in Rat Brain following the Early Post-Stroke Period
by
Ivan B. Filippenkov, Julia A. Remizova, Vasily V. Stavchansky, Alina E. Denisova, Leonid V. Gubsky, Nikolay F. Myasoedov, Svetlana A. Limborska and Lyudmila V. Dergunova
Cited by 1 | Viewed by 1213
Abstract
Ischemic stroke is an acute local decrease in cerebral blood flow due to a thrombus or embolus. Of particular importance is the study of the genetic systems that determine the mechanisms underlying the formation and maintenance of a therapeutic window (a time interval
[...] Read more.
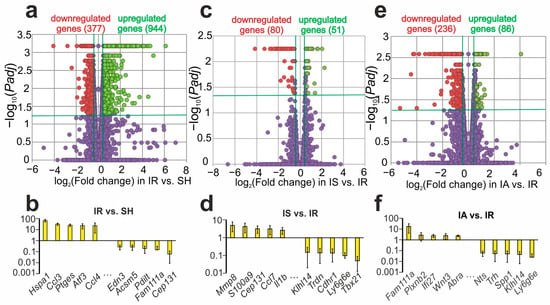
Ischemic stroke is an acute local decrease in cerebral blood flow due to a thrombus or embolus. Of particular importance is the study of the genetic systems that determine the mechanisms underlying the formation and maintenance of a therapeutic window (a time interval of up to 6 h after a stroke) when effective treatment can be provided. Here, we used a transient middle cerebral artery occlusion (tMCAO) model in rats to study two synthetic derivatives of adrenocorticotropic hormone (ACTH). The first was ACTH(4-7)PGP, which is known as Semax. It is actively used as a neuroprotective drug. The second was the ACTH(6-9)PGP peptide, which is elucidated as a prospective agent only. Using RNA-Seq analysis, we revealed hundreds of ischemia-related differentially expressed genes (DEGs), as well as 131 and 322 DEGs related to the first and second peptide at 4.5 h after tMCAO, respectively, in dorsolateral areas of the frontal cortex of rats. Furthermore, we showed that both Semax and ACTH(6-9)PGP can partially prevent changes in the immune- and neurosignaling-related gene expression profiles disturbed by the action of ischemia at 4.5 h after tMCAO. However, their different actions with regard to predominantly immune-related genes were also revealed. This study gives insight into how the transcriptome depends on the variation in the structure of the related peptides, and it is valuable from the standpoint of the development of measures for early post-stroke therapy.
Full article
►▼
Show Figures
Open AccessArticle
Divergent Transcriptomic Effects of Allopregnanolone in Postpartum Depression
by
Sarah A. Rudzinskas, Maria A. Mazzu, Crystal Edler Schiller, Samantha Meltzer-Brody, David R. Rubinow, Peter J. Schmidt and David Goldman
Cited by 1 | Viewed by 1575
Abstract
Brexanolone, a formulation of the neurosteroid allopregnanolone (ALLO), is approved for treating postpartum depression (PPD) and is being investigated for therapeutic efficacy across numerous neuropsychiatric disorders. Given ALLO’s beneficial effects on mood in women with PPD compared to healthy control women, we sought
[...] Read more.
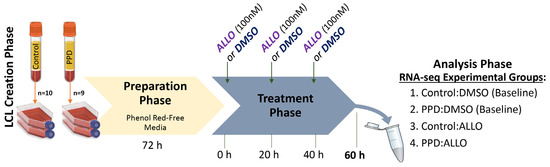
Brexanolone, a formulation of the neurosteroid allopregnanolone (ALLO), is approved for treating postpartum depression (PPD) and is being investigated for therapeutic efficacy across numerous neuropsychiatric disorders. Given ALLO’s beneficial effects on mood in women with PPD compared to healthy control women, we sought to characterize and compare the cellular response to ALLO in women with (
n = 9) or without (
n = 10, i.e., Controls) past PPD, utilizing our previously established patient-derived lymphoblastoid cell lines (LCLs). To mimic in vivo PPD ALLO-treatment, LCLs were exposed to ALLO or DMSO vehicle for 60 h and RNA-sequenced to detect differentially expressed genes (DEGs, p
nominal < 0.05). Between ALLO-treated Control and PPD LCLs, 269 DEGs were identified, including Glutamate Decarboxylase 1 (
GAD1), which was decreased 2-fold in PPD. Network analysis of PPD:ALLO DEGs revealed enriched terms related to synaptic activity and cholesterol biosynthesis. Within-diagnosis analyses (i.e., DMSO vs. ALLO) detected 265 ALLO-induced DEGs in Control LCLs compared to only 98 within PPD LCLs, with just 11 DEGs overlapping. Likewise, the gene ontologies underlying ALLO-induced DEGs in PPD and Control LCLs were divergent. These data suggest that ALLO may activate unique and opposing molecular pathways in women with PPD, which may be tied to its antidepressant mechanism.
Full article
►▼
Show Figures
Open AccessArticle
Maternal Immune Activation and Enriched Environments Impact B2 SINE Expression in Stress Sensitive Brain Regions of Rodent Offspring
by
Troy A. Richter, Ariel A. Aiken, Madeline J. Puracchio, Ismael Maganga-Bakita and Richard G. Hunter
Viewed by 1312
Abstract
Early life stress (ELS) can have wide-spread neurodevelopmental effects with support accumulating for the idea that genomic mechanisms may induce lasting physiological and behavioral changes following stress exposure. Previous work found that a sub-family of transposable elements, SINEs, are repressed epigenetically after acute
[...] Read more.
Early life stress (ELS) can have wide-spread neurodevelopmental effects with support accumulating for the idea that genomic mechanisms may induce lasting physiological and behavioral changes following stress exposure. Previous work found that a sub-family of transposable elements, SINEs, are repressed epigenetically after acute stress. This gives support to the concept that the mammalian genome may be regulating retrotransposon RNA expression allowing for adaptation in response to environmental challenges, such as maternal immune activation (MIA). Transposon (TE) RNAs are now thought to work at the epigenetic level and to have an adaptive response to environmental stressors. Abnormal expression of TEs has been linked to neuropsychiatric disorders like schizophrenia, which is also linked to maternal immune activation. Environmental enrichment (EE), a clinically utilized intervention, is understood to protect the brain, enhance cognitive performance, and attenuate responses to stress. This study examines the effects of MIA on offspring B2 SINE expression and further, the impact that EE, experienced throughout gestation and early life, may have in conjunction with MIA during development. Utilizing RT-PCR to quantify the expression of B2 SINE RNA in the juvenile brain of MIA exposed rat offspring, we found dysregulation of B2 SINE expression associated with MIA in the prefrontal cortex. For offspring experiencing EE, the prefrontal cortex exhibited an attenuation of the MIA response observed in standard housed animals. Here, the adaptive nature of B2 is observed and thought to be aiding in the animal’s adaptation to stress. The present changes indicate a wide-spread stress-response system adaptation that impacts not only changes at the genomic level but potentially observable behavioral impacts throughout the lifespan, with possible translational relevance to psychotic disorders.
Full article
►▼
Show Figures
Open AccessArticle
Exome Array Analysis of 9721 Ischemic Stroke Cases from the SiGN Consortium
by
Huichun Xu, Kevin Nguyen, Brady J. Gaynor, Hua Ling, Wei Zhao, Patrick F. McArdle, Timothy D. O’Connor, O. Colin Stine, Kathleen A. Ryan, Megan Lynch, Jennifer A. Smith, Jessica D. Faul, Yao Hu, Jeffrey W. Haessler, Myriam Fornage, Charles Kooperberg, on behalf of the Trans-Omics for Precision Medicine (TOPMed) Stroke Working Group, James A. Perry, Charles C. Hong, John W. Cole, Elizabeth Pugh, Kimberly Doheny, Sharon L. R. Kardia, David R. Weir, Steven J. Kittner and Braxton D. Mitchelladd
Show full author list
remove
Hide full author list
| Viewed by 1910
Abstract
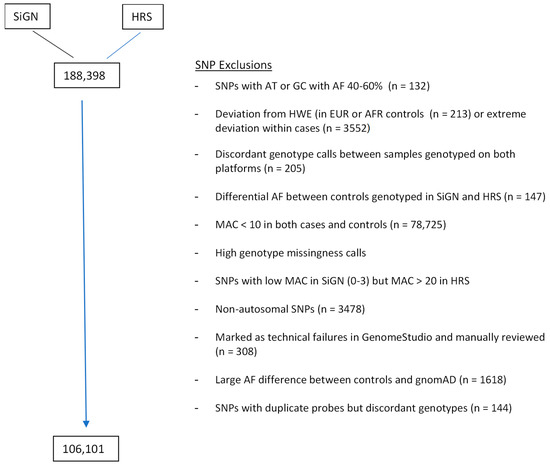
Recent genome wide association studies have identified 89 common genetic variants robustly associated with ischemic stroke and primarily located in non-coding regions. To evaluate the contribution of coding variants, which are mostly rare, we performed an exome array analysis on 106,101 SNPs for
[...] Read more.
Recent genome wide association studies have identified 89 common genetic variants robustly associated with ischemic stroke and primarily located in non-coding regions. To evaluate the contribution of coding variants, which are mostly rare, we performed an exome array analysis on 106,101 SNPs for 9721 ischemic stroke cases from the SiGN Consortium, and 12,345 subjects with no history of stroke from the Health Retirement Study and SiGN consortium. We identified 15 coding variants significantly associated with all ischemic stroke at array-wide threshold (i.e.,
p < 4.7 × 10
−7), including two common SNPs in
ABO that have previously been associated with stroke. Twelve of the remaining 13 variants were extremely rare in European Caucasians (MAF < 0.1%) and the associations were driven by African American samples. There was no evidence for replication of these associations in either TOPMed Stroke samples (
n = 5613 cases) or UK Biobank (
n = 5874 stroke cases), although power to replicate was very low given the low allele frequencies of the associated variants and a shortage of samples from diverse ancestries. Our study highlights the need for acquiring large, well-powered diverse cohorts to study rare variants, and the technical challenges using array-based genotyping technologies for rare variant genotyping.
Full article
►▼
Show Figures
Open AccessReview
Intracerebral Hemorrhage Genetics
by
Aleksandra Ekkert, Aleksandra Šliachtenko, Algirdas Utkus and Dalius Jatužis
Cited by 6 | Viewed by 2671
Abstract
Intracerebral hemorrhage (ICH) is a devastating type of stroke, frequently resulting in unfavorable functional outcomes. Up to 15% of stroke patients experience ICH and approximately half of those have a lethal outcome within a year. Considering the huge burden of ICH, timely prevention
[...] Read more.
Intracerebral hemorrhage (ICH) is a devastating type of stroke, frequently resulting in unfavorable functional outcomes. Up to 15% of stroke patients experience ICH and approximately half of those have a lethal outcome within a year. Considering the huge burden of ICH, timely prevention and optimized treatment strategies are particularly relevant. Nevertheless, ICH management options are quite limited, despite thorough research. More and more trials highlight the importance of the genetic component in the pathogenesis of ICH. Apart from distinct monogenic disorders of familial character, mostly occurring in younger subjects, there are numerous polygenic risk factors, such as hypertension, neurovascular inflammation, disorders of lipid metabolism and coagulation cascade, and small vessel disease. In this paper we describe gene-related ICH types and underlying mechanisms. We also briefly discuss the emerging treatment options and possible clinical relevance of the genetic findings in ICH management. Although existing data seems of more theoretical and scientific value so far, a growing body of evidence, combined with rapidly evolving experimental research, will probably serve clinicians in the future.
Full article