Water and Wastewater Treatment Technologies
Share This Topical Collection
Editor
 Prof. Dr. Susana Rodriguez-Couto
Prof. Dr. Susana Rodriguez-Couto
 Prof. Dr. Susana Rodriguez-Couto
Prof. Dr. Susana Rodriguez-Couto
E-Mail
Website
Collection Editor
Department of Separation Science, LUT School of Engineering Science, LUT University, Sammonkatu 12, FI-50130 Mikkeli, Finland
Interests: microbiology; bioelectrochemical fuel cells; bioengineering chemical engineering; environmental science; water science and technology
Topical Collection Information
Dear Colleagues,
Growing populations, urbanization and industrialization all generate large volumes of wastewater containing hazardous pollutants, most of which are recalcitrant, causing their accumulation and persistence in the environment and placing wildlife and human health at risk. Therefore, the removal of such compounds from wastewater before its discharge into the environment is an urgent requirement, conventional treatment techniques presenting the following limitations: a low efficiency, high cost and the generation of toxic by-products. This has driven the search for novel efficient and environmentally friendly technologies, the current book focusing on stated emerging technologies, as well as the modification of conventional technologies for water and wastewater treatment. In addition, the reuse of water and the recovery of valuable compounds from wastewater are considered to promote the transition from a lineal to a circular economy in wastewater treatment, complying with the United Nations’ sustainable development goals (SDGs) for 2030.
Prof. Dr. Susana Rodríguez-Couto
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Clean Technologies is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- wastewater
- hazardous pollutants
- sustainability
- circular economy
- water reuse
- recovery of valuable compounds
Published Papers (9 papers)
Open AccessArticle
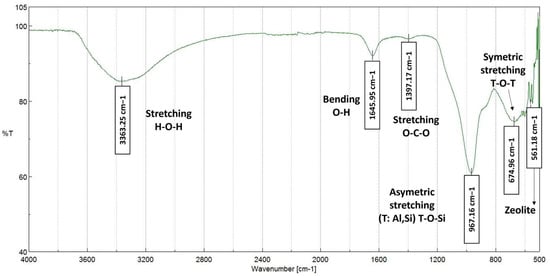
Ammonium Removal in Wastewater Treatments by Adsorbent Geopolymer Material with Granite Wastes: Full-Scale Validation
by
M. Otero, L. Freire, S. Gómez-Cuervo and C. Ávila
Viewed by 1200
Abstract
Elevated ammonium (NH
4+) concentrations in untreated waterways contribute to eutrophication and dissolved oxygen depletion. Geopolymer (GP) materials are introduced as sustainable, straightforward operation and low-cost option for pollutant adsorption through ion exchange mechanism. In the present study, a porous metakaolin-based
[...] Read more.
Elevated ammonium (NH
4+) concentrations in untreated waterways contribute to eutrophication and dissolved oxygen depletion. Geopolymer (GP) materials are introduced as sustainable, straightforward operation and low-cost option for pollutant adsorption through ion exchange mechanism. In the present study, a porous metakaolin-based geopolymer with granite waste additions was synthetized, characterised and validated as adsorbent material for NH
4+ pollution in water. At this point, treatments to reduce GP alkalis leaching were also considered to comply with the water discharge regulations. The adsorption mechanism was analysed by Redlich-Peterson isotherm model concluding that NH
4+ was disposed on the GP surface as a monolayer with strong physical-chemical attraction between molecules. Kinetics of the process followed the Weber-Morris rate equation being the intraparticle diffusion the limiting process. Continuous experiments at lab-scale suggested a maximum removal of 97% during the first hours and an adsorption capacity (q) of 25.24 mg/g. Additionally, as a main novelty of the work, the GP was validated in a full-scale pilot plant monitoring pH, electrical conductivity and NH
4+ concentration. The obtained data revealed that the GP is high selective in a real wastewater stream and removed 81% of NH
4+, higher adsorption values than those reported for natural and some synthetic zeolites.
Full article
►▼
Show Figures
Open AccessArticle
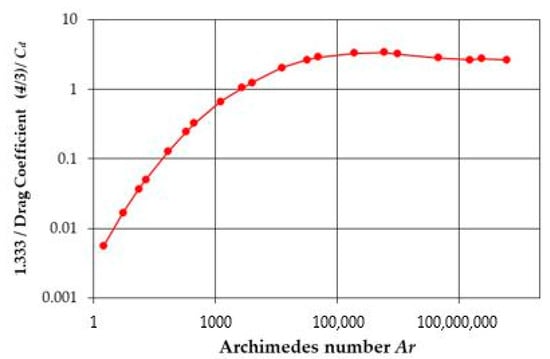
Mathematical Modeling of Particle Terminal Velocity for Improved Design of Clarifiers, Thickeners and Flotation Devices for Wastewater Treatment
by
Dario Friso
Viewed by 1234
Abstract
The prediction of the terminal velocity of a single spherical particle is essential to realize mathematical modeling useful for the design and adjustment of separators used in wastewater treatment. For non-spherical and non-single particles, terminal velocity can be traced back to that of
[...] Read more.
The prediction of the terminal velocity of a single spherical particle is essential to realize mathematical modeling useful for the design and adjustment of separators used in wastewater treatment. For non-spherical and non-single particles, terminal velocity can be traced back to that of single spheres using coefficients and Kynch’s theory, respectively. Because separation processes can involve small or large particles and can be carried out using gravity, as with clarifiers/thickeners, or by centrifugation in centrifuges where the acceleration can exceed 10,000×
g, the Reynolds number of the particle can be highly variable, ranging from 0.1 to 200,000. The terminal velocity depends on the drag coefficient, which depends, in turn, on the Reynolds number containing the terminal velocity. Because of this, to find the terminal velocity formula, it is preferable to look first for a relationship between the drag coefficient and the Archimedes number which does not contain the terminal velocity. Formulas already exist expressing the relationship between the drag coefficient and the Archimedes number, from which the relationship between the terminal velocity and the Archimedes number may be derived. To improve the accuracy obtained by these formulas, a new relationship was developed in this study, using dimensional analysis, which is valid for Reynolds number values between 0.1 and 200,000. The resulting mean relative difference, compared to the experimental standard drag curve, was only 1.44%. This formula was developed using the logarithms of dimensionless numbers, and the unprecedented accuracy obtained with this method suggested that an equally accurate formula for the drag coefficient could also be obtained with respect to the Reynolds number. Again, the resulting level of accuracy was unprecedentedly high, with a mean relative difference of 1.77% for Reynolds number values between 0.1 and 200,000.
Full article
►▼
Show Figures
Open AccessArticle
Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species
by
Franco Hernan Gomez, Maria Cristina Collivignarelli, Ahmed Mohammad Nafea Masoud, Marco Carnevale Miino, Kelly Cristina Torres, Jesus Antonio Quintero, Sabrina Sorlini and Mentore Vaccari
Viewed by 1928
Abstract
Mining wastewater (MWW) can contain mercury in high concentrations. In this study, four autochthonous aquatic plant species (
Eichhornia Crassipes—EC,
Marsilea Quadrifolia—MQ,
Ludwigia Helminthorrhiza—LH, and
Lemna Minor—LM) were identified and tested for phytoaccumulation of total mercury (THg). To better
[...] Read more.
Mining wastewater (MWW) can contain mercury in high concentrations. In this study, four autochthonous aquatic plant species (
Eichhornia Crassipes—EC,
Marsilea Quadrifolia—MQ,
Ludwigia Helminthorrhiza—LH, and
Lemna Minor—LM) were identified and tested for phytoaccumulation of total mercury (THg). To better study the accumulation phenomenon and macrophyte responses, this work has been divided into three phases, and pilot-scale reactors have been used to simulate real conditions. The results highlighted that, in case of 15 µg
THg,fed, the bioconcentration factor (BCF) was significantly higher in EC (19.04) and LH (18.41) with respect to MQ and LM (almost six times and two times higher, respectively). EC granted the best results in terms of THg accumulation (50.90%) and lower evapotranspiration of THg phenomenon with respect to LH. A significant decrease of the BCF (from 23.45 to 21.98) and an increase of the TF (from 0.23 up to 0.73) after 42 d highlighted that a breaking-time in terms of THg accumulation was reached due to the deterioration of the roots. In terms of the kinetics of THg removal by bioaccumulation, an HLT of 69.31 d was found, which is more than the breaking-time of the EC system, proving that a periodic replacement of exhausted macrophytes is required to obtain a higher percentage of THg removal.
Full article
►▼
Show Figures
Open AccessArticle
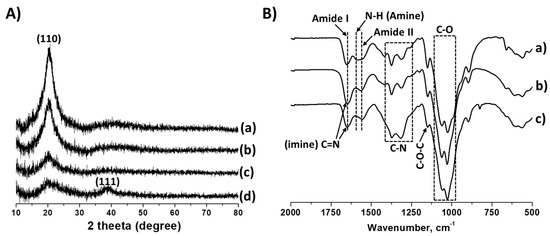
Efficient Adsorption and Catalytic Reduction of Phenol Red Dye by Glutaraldehyde Cross-Linked Chitosan and Its Ag-Loaded Catalysts: Materials Synthesis, Characterization and Application
by
Chiara Concetta Siciliano, Van Minh Dinh, Paolo Canu, Jyri-Pekka Mikkola and Santosh Govind Khokarale
Cited by 1 | Viewed by 2156
Abstract
In this study, glutaraldehyde cross-linked chitosan support, as well as the catalysts obtained after loading Ag metal (Ag/Chitosan), were synthesised and applied for adsorption and reduction of phenol red dye in an aqueous solution. The Ag/chitosan catalysts were characterised by X-ray diffraction (XRD),
[...] Read more.
In this study, glutaraldehyde cross-linked chitosan support, as well as the catalysts obtained after loading Ag metal (Ag/Chitosan), were synthesised and applied for adsorption and reduction of phenol red dye in an aqueous solution. The Ag/chitosan catalysts were characterised by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR) and inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis techniques. The catalytic reduction and adsorption performance of phenol red dye with Ag/chitosan and cross-linked chitosan, respectively, was performed at ambient reaction conditions. The reduction of dye was carried out using sodium borohydride (NaBH
4) as the reducing agent, while the progress of adsorption and reduction study was monitored with ultraviolet-visible (UV-vis) spectrophotometry technique. The reduction of the phenol red dye varied with the amount of catalyst, the concentration of NaBH
4, Ag metal loading, reaction temperature, phenol red dye concentration and initial
pH of the dye solution. The dye solution with a nearly-neutral
pH (6.4) allowed efficient adsorption of the dye, while acidic (
pH = 4) and alkaline (
pH = 8, 11, 13.8) solutions showed incomplete or no adsorption of dye. The reusability of the Ag/chitosan catalyst was applied for the complete reduction of the dye, where no significant loss of catalytic activity was observed. Hence, the applicability of cross-linked chitosan and Ag/catalyst was thus proven for both adsorption and reduction of phenol red dye in an aqueous solution and can be applied for industrial wastewater treatment.
Full article
►▼
Show Figures
Open AccessReview
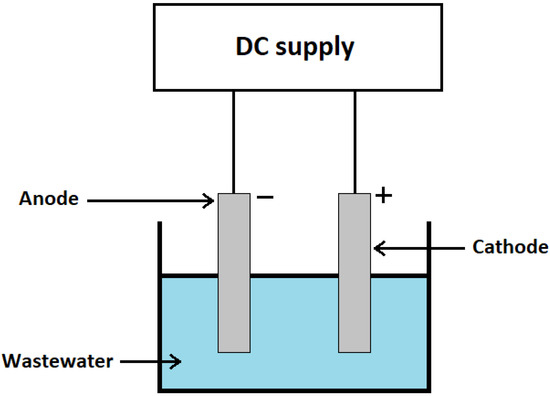
The Combined Implementation of Electrocoagulation and Adsorption Processes for the Treatment of Wastewaters
by
Nuno S. Graça and Alírio E. Rodrigues
Cited by 8 | Viewed by 4289
Abstract
Effluent treatment and reuse are essential in order to address the global problem of water scarcity. Additionally, improving the quality of treated wastewater is necessary to reduce its adverse effects on natural water resources and, consequently, on human health. Electrocoagulation and adsorption have
[...] Read more.
Effluent treatment and reuse are essential in order to address the global problem of water scarcity. Additionally, improving the quality of treated wastewater is necessary to reduce its adverse effects on natural water resources and, consequently, on human health. Electrocoagulation and adsorption have been successfully applied separately to treat different wastewaters. Each method has unique benefits, drawbacks, and parameters that affect the effectiveness of treatment. A review of both processes, including their theoretical principles, the effect of operating conditions, and practical applications, is presented to provide an overview of their capabilities. The combination of electrocoagulation and adsorption in a combined process can be used to amplify the advantages of each process while mitigating their limitations. In the present work, the combined process is analyzed in terms of its principles, applications, and integration in a circular economy model.
Full article
►▼
Show Figures
Open AccessArticle
Circular Water Economy in the EU: Findings from Demonstrator Projects
by
Yahya Qtaishat, Jan Hofman and Kemi Adeyeye
Cited by 11 | Viewed by 4650
Abstract
Circular economy (CE) for water aims to maximise value derived from water, processes, and practices. As a result, the recovery of wastewater and renewable water resources is used to offset the exploitation and impact of abstracting new water resources. New regulations such as
[...] Read more.
Circular economy (CE) for water aims to maximise value derived from water, processes, and practices. As a result, the recovery of wastewater and renewable water resources is used to offset the exploitation and impact of abstracting new water resources. New regulations such as the new circular economy action plan by the European Commission are emerging to promote circularity within the Green Deal agenda. However, there is still a need for research and practical insights into the interaction and integration of CE for water within existing policies and regulations, and its practical application specifically at the project level. This paper presents findings from demonstrator cases used to explore the opportunities and constraints in the policy, process, and procedural frameworks that govern water circularity in important sectors in Europe. Desk reviews are used to examine and compare European legislation against national and regional legislative frameworks within the different member states. Interviews and demonstrator project feedback enabled the exploration of the policy and value constraints at the project level. The findings provide unique insights into the policy and legislative enablers for and barriers to implementing CE for water in key sectors and specifically at the project level. The paper concludes with a five-point route map for new and revised policies and regulations targeting improved uptake of circular water technologies in Europe.
Full article
►▼
Show Figures
Open AccessArticle
Efficient Management of Sewage Sludge from Urban Wastewaters with the Addition of Inorganic Waste: Focus on Rheological Properties
by
Andreia F. Santos, Abel G. M. Ferreira and Margarida J. Quina
Cited by 4 | Viewed by 2479
Abstract
Sewage sludge (SS) from urban wastewater treatment is still an environmental, economic, and social problem. Current SS management is not consensual, and more alternatives are required to recover some valuable compounds, such as nutrients and organic matter. This study investigates the use of
[...] Read more.
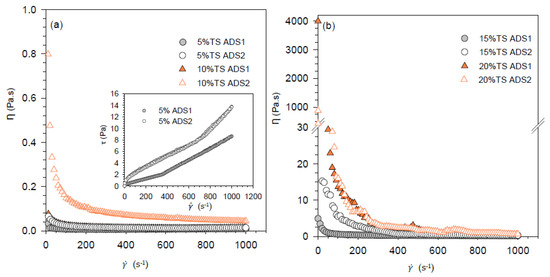
Sewage sludge (SS) from urban wastewater treatment is still an environmental, economic, and social problem. Current SS management is not consensual, and more alternatives are required to recover some valuable compounds, such as nutrients and organic matter. This study investigates the use of green liquor dregs from the pulp and paper industry—GLDs—as an adjuvant of drying, to develop a product for agronomic applications, focusing on the rheological behavior. The rheological properties were assessed for anaerobically digested sludge (ADS). The limit viscosity of raw ADS was about 0.005 Pa·s in the case of 5% TSs (total solids) increasing to 0.51 Pa·s for 20% TSs. From the oscillatory tests, the ideal viscous flow below 10% TSs was observed, whereas a viscoelastic–solid behavior was detected for a higher concentration of TSs. The addition of GLDs to the ADS reduced the consistency index, reducing the shear resistance of the material. Rheological assays showed that GLDs may facilitate sludge handling (e.g., extrusion) from the dewatering unit to the dryer. Overall, the addition of GLDs to ADS showed to be a viable option for drying and subsequent soil application. Reusing both residues promote the transition from a linear to a circular economy in the wastewater treatment sector.
Full article
►▼
Show Figures
Open AccessArticle
Enhanced Sewage Sludge Drying with a Modified Solar Greenhouse
by
Alice Sorrenti, Santo Fabio Corsino, Francesco Traina, Gaspare Viviani and Michele Torregrossa
Viewed by 3732
Abstract
This work reports the results obtained with an innovative configuration of a closed-static solar greenhouse for sludge drying. The novelty of the solar greenhouse configuration consisted in using a forced ventilation system to provide hot air for sludge drying and the utilization of
[...] Read more.
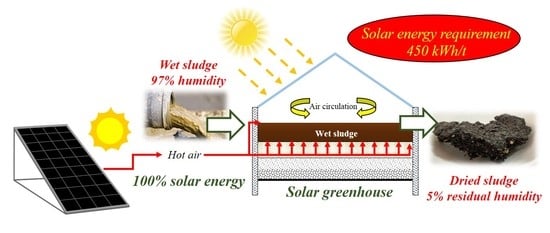
This work reports the results obtained with an innovative configuration of a closed-static solar greenhouse for sludge drying. The novelty of the solar greenhouse configuration consisted in using a forced ventilation system to provide hot air for sludge drying and the utilization of solar irradiation for energy supply. Wet sewage sludge (97% humidity) was successfully dried up to a residual humidity close to 5% after 25 days during wintertime. The increase of the airflow rate supplied under the sludge bed improved the sludge drying rate. Moreover, the fraction of volatile suspended solids decreased from 70% to 41% after 13 days, indicating that air supply promoted the simultaneous stabilization of the sludge as a side-effect to the drying process. Overall, the specific energy consumption per ton of evaporated water was estimated to approximately 450 kWh/t, resulting in about 55% of energy demand lower than a conventional thermal drying system, while using only free solar energy. The achieved high weight reduction of up to 99% implies a noticeable reduction of the excess sludge handling costs, indicating that solar greenhouse drying is a highly interesting opportunity for sludge drying in medium-small sized WWTPs.
Full article
►▼
Show Figures
Open AccessArticle
Sorption of 71 Pharmaceuticals to Powder Activated Carbon for Improved Wastewater Treatment
by
Maritha Hörsing, Henrik Rasmus Andersen, Roman Grabic, Jes la Cour Jansen and Anna Ledin
Viewed by 2356
Abstract
In this study, sorption distribution coefficients were determined for 71 pharmaceuticals, aiming to describe their sorption behavior to powder activated carbon (PAC). The data are expected to be applied when designing and upgrading wastewater treatment plants (WWTP) for improved removal of pharmaceuticals by
[...] Read more.
In this study, sorption distribution coefficients were determined for 71 pharmaceuticals, aiming to describe their sorption behavior to powder activated carbon (PAC). The data are expected to be applied when designing and upgrading wastewater treatment plants (WWTP) for improved removal of pharmaceuticals by applying sorption to PAC as an additional removal technique. Sorption isotherms were determined for the pharmaceuticals over a concentration interval covering a wide range from 0.08 to 10 µg/L using PAC at a concentration of 10 mg/L. The best fitted sorption isotherms were used to calculate the distribution coefficients (
Kd) and these were applied to estimate that the PAC doses needed to achieve a target concentration of 10 ng/L in the effluent. A target concentration was used since neither discharge limit values nor environmental quality standards in general have been defined for these compounds. Using a %-removal approach does not guarantee achievement of concentrations low enough to protect the water ecosystems. Some of the pharmaceuticals will be reduced by the addition of small amounts of PAC. Examples are atenolol, carbamazepine, citalopram, codeine, fluoxetine and ibuprofen. For others, e.g., oxazepam, an alternative treatment has to be considered since the requested dose is too high to be realistic for a target concentration of 10 ng/L.
Full article
►▼
Show Figures