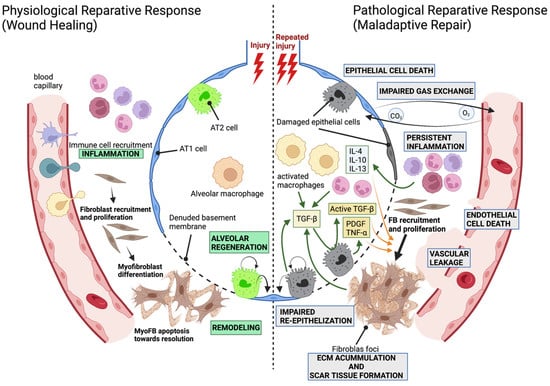
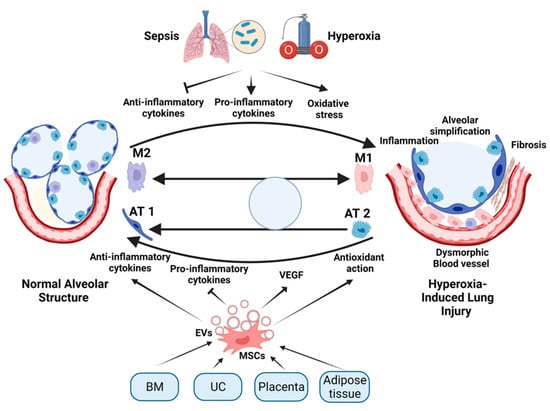
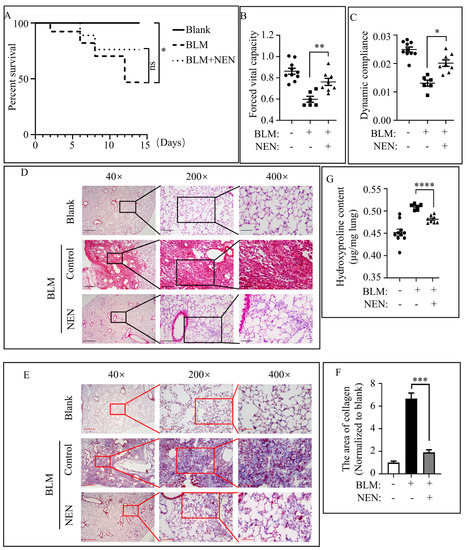
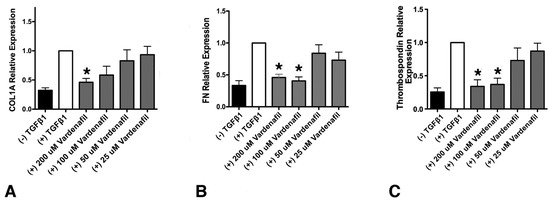
Pulmonary Fibrosis and Cell Therapy
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Microenvironment".
Viewed by 29326Editor
Interests: pulmonary fibrosis; cell therapy; type II alveolar cells; pulmonary macrophages; fibroblasts; lung cells; cell cultures; lung surfactant; lung inflammation; experimental models of lung disease
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
During these last two decades, cell therapies for the treatment of pulmonary fibrosis have been considered a promising treatment. Since then, different types of stem cells or lung progenitor cells have been evaluated, mostly in animal models and only in a few human clinical trials. However, although both preclinical and human clinical data support the safety of cell therapies, they have not been translated into routine clinical practice. The reasons are diverse, since many key unanswered questions remain, such as doubts about the type of cells to be transplanted, the origin of these cells (autologous or heterologous), the route of administration (intravenous or endotracheal), the dose, the best time for transplantation and others. Additionally, the concern about side effects, such as unwanted differentiation of the grafted stem cells, cannot be forgotten. In addition to answering these questions, it is essential to know the mechanisms by which cell therapies exert their positive effects. Recent studies indicate that most of the positive effects can be attributed to the cellular secretome, composed of free soluble proteins and extracellular vesicles. The use of the secretome would avoid the side effects associated with cells, as well as the difficulty in obtaining and expanding them. Therefore, it is not only important to answer these questions, but also to know the mechanisms by which cell therapies exert their positive effects. All of this knowledge would help consolidate cell therapies in clinical practice.
This Topical Collection aims to summarize the updated knowledge on different cell therapies and the mechanisms involved in their potential therapeutic effect in pulmonary fibrosis.
The Topical Collection, entitled "Pulmonary Fibrosis and Cell Therapy", will include both review and original research papers covering key topics related to different cell therapies for pulmonary fibrosis, such as (1) different types of stem cells, induced pluripotent stem cells (iPSC), non-derived and derived to pulmonary cells; (2) lung progenitor cells; (3) other types of pulmonary cells; (4) cell secretome; (5) cell–cell interactions; (6) investigating mechanisms of lung tissue regeneration, cell engraftment or cell secretome. We hope that our readers from various disciplines will benefit from this Topical Collection, which will serve as a gateway to the amazing world of cell therapies for fibrotic diseases.
We look forward to your contributions.
Dr. Anna Serrano-Mollar
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- stem and progenitor cells
- induced pluripotent stem cells (iPSC)
- induced pluripotent stem cell-derived pulmonary cells (iPSC-PC)
- spheroids
- organoids
- cell secretome
- cell dose
- cell route of administration
- cell-derived drugs
- molecular mechanism
- tissue regeneration mechanism
2022
Jump to: 2021
2021
Jump to: 2022
Planned Papers
The below list represents only planned manuscripts. Some of these manuscripts have not been received by the Editorial Office yet. Papers submitted to MDPI journals are subject to peer-review.
Title: Single Cell Deconvolution of Pulmonary Fibrosis(tentative title)
Authors: Amir Abdollahi; Jennifer Furkel; Mahmoud Moustafa; Max Knoll
Affiliation: Division of Molecular and Translational Radiation Oncology Heidelberg Faculty of Medicine (MFHD) Heidelberg University Hospital (UKHD) Heidelberg Ion-Beam Therapy Center (HIT) Im Neuenheimer Feld 450 Director: Radiation Oncology Programs Head: Personalized Radiation Oncology (NCT-PRO) National Center for Tumor Disease (NCT) Im Neuenheimer Feld 460 Clinical Cooperation Unit Translational Radiation Oncology (E210) National Coordinator: Radiation Oncology Program German Cancer Consortium (DKTK) German Cancer Research Center (DKFZ) Im Neuenheimer Feld 223 69120 Heidelberg Germany
Abstract: Our manuscript will describe pitfalls and the methodological developments needed to achieve a comprehensive cellular diversity representation in normal vs. fibrotic lungs via extrocoporal perfusion of two preclinical mouse lung models etc. and 10X NGS single cell transcriptomics.