Stem Cell Therapies for Treating Diabetes
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 33877
Share This Topical Collection
Editors
 Dr. Jun Shirakawa
Dr. Jun Shirakawa
 Dr. Jun Shirakawa
Dr. Jun Shirakawa
E-Mail
Website
Collection Editor
Yokohama City University, Yokohama, Japan
Interests: pancreatic beta cells; pancreatic islets; diabetes; obesity; metabolism
 Dr. Adrian Kee Keong Teo
Dr. Adrian Kee Keong Teo
 Dr. Adrian Kee Keong Teo
Dr. Adrian Kee Keong Teo
E-Mail
Website
Collection Editor
Institute of Molecular and Cell Biology (IMCB), A*STAR; Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Interests: stem cells; diabetes; pancreas; islet; beta cell; metabolism; complications
Topical Collection Information
As of 2019, more than 463 million people in the world live with diabetes. This is a serious threat to global health and is certainly a big issue in terms of economic impact. In the past decades, there has been active research into using stem cell therapy to treat diabetes, such as type 1 or type 2 diabetes. In this Topical Collection, we define stem cells broadly as unique immature cells that harbor an immense potential to differentiate into various cell types of the body. They can originate from tissues such as the human embryo, placenta, umbilical cord, bone marrow, or blood cells. Since the discovery and identification of these various types of stem cells in the human body, there have been immense efforts to generate human pancreatic islets or insulin-producing beta cells from these varied stem cells, for the purpose of restoring euglycemia. More recently, methodologies to make human islet or beta-like cells have matured significantly. It appears that the human stem cell field is very close to using stem cell-derived islets or beta cells to treat or even cure diabetes. In view of these significant advances and 2021 being the 100th anniversary for the discovery of insulin, we decided to commission this timely Topical Collection, in the hope that stem cell-based therapy will pave the way for the next century in moving the field closer toward an eventual cure for diabetes.
Dr. Adrian Kee Keong Teo
Dr. Jun Shirakawa
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- stem cell
- cell therapy
- clinical trial
- regenerative medicine
- human
- pancreas
- islet
- beta cell
- insulin
- diabetes
Published Papers (8 papers)
Open AccessArticle
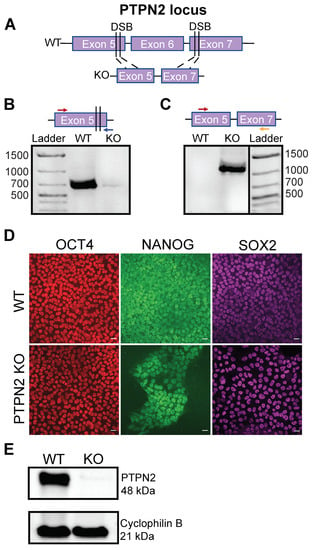
Stem-Cell-Derived β-Like Cells with a Functional PTPN2 Knockout Display Increased Immunogenicity
by
Taylor M. Triolo, J. Quinn Matuschek, Roberto Castro-Gutierrez, Ali H. Shilleh, Shane P. M. Williams, Maria S. Hansen, Kristen McDaniel, Jessie M. Barra, Aaron Michels and Holger A. Russ
Cited by 2 | Viewed by 2158
Abstract
Type 1 diabetes is a polygenic disease that results in an autoimmune response directed against insulin-producing beta cells.
PTPN2 is a known high-risk type 1 diabetes associated gene expressed in both immune- and pancreatic beta cells, but how genes affect the development of
[...] Read more.
Type 1 diabetes is a polygenic disease that results in an autoimmune response directed against insulin-producing beta cells.
PTPN2 is a known high-risk type 1 diabetes associated gene expressed in both immune- and pancreatic beta cells, but how genes affect the development of autoimmune diabetes is largely unknown. We employed CRISPR/Cas9 technology to generate a functional knockout of
PTPN2 in human pluripotent stem cells (hPSC) followed by differentiating stem-cell-derived beta-like cells (sBC) and detailed phenotypical analyses. The differentiation efficiency of
PTPN2 knockout (PTPN2 KO) sBC is comparable to wild-type (WT) control sBC. Global transcriptomics and protein assays revealed the increased expression of HLA Class I molecules in PTPN2 KO sBC at a steady state and upon exposure to proinflammatory culture conditions, indicating a potential for the increased immune recognition of human beta cells upon differential
PTPN2 expression. sBC co-culture with autoreactive preproinsulin-reactive T cell transductants confirmed increased immune stimulations by PTPN2 KO sBC compared to WT sBC. Taken together, our results suggest that the dysregulation of
PTPN2 expression in human beta cell may prime autoimmune T cell reactivity and thereby contribute to the development of type 1 diabetes.
Full article
►▼
Show Figures
Open AccessArticle
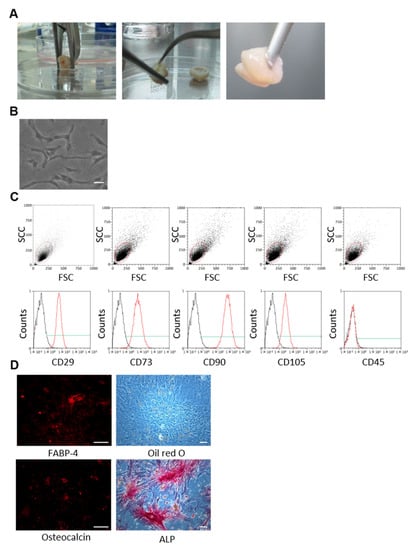
Sustainable Effects of Human Dental Pulp Stem Cell Transplantation on Diabetic Polyneuropathy in Streptozotocine-Induced Type 1 Diabetes Model Mice
by
Masaki Hata, Maiko Omi, Yasuko Kobayashi, Nobuhisa Nakamura, Megumi Miyabe, Mizuho Ito, Tasuku Ohno, Yuka Imanishi, Tatsuhito Himeno, Hideki Kamiya, Jiro Nakamura, Hitoshi Miyachi, Shogo Ozawa, Ken Miyazawa, Akio Mitani, Toru Nagao, Shigemi Goto, Jun Takebe, Tatsuaki Matsubara and Keiko Naruse
Cited by 9 | Viewed by 2447
Abstract
Dental pulp stem cells (DPSCs) are suitable for use in regenerative medicine. Cryopreserved human DPSCs (hDPSCs) ameliorate diabetic polyneuropathy, and the effects of hDPSC transplantation are related to VEGF and NGF secretion. This study evaluated the long-term effects of a single transplantation of
[...] Read more.
Dental pulp stem cells (DPSCs) are suitable for use in regenerative medicine. Cryopreserved human DPSCs (hDPSCs) ameliorate diabetic polyneuropathy, and the effects of hDPSC transplantation are related to VEGF and NGF secretion. This study evaluated the long-term effects of a single transplantation of hDPSCs on diabetic polyneuropathy. hDPSCs were obtained from human third molars extracted for orthodontic treatment, which were then transplanted into the unilateral hindlimb skeletal muscles 8 weeks after streptozotocin injection in nude mice. The effects of hDPSC transplantation were analyzed at 16 weeks post-transplantation. DPSC transplantation significantly improved delayed nerve conduction velocity, decreased blood flow, and increased sensory perception thresholds. Furthermore, the hDPSC-conditioned medium promoted the neurite outgrowth of dorsal root ganglion neurons. In conclusion, the therapeutic effects of hDPSC transplantation with a single injection last for prolonged periods and may be beneficial in treating long-term diabetic polyneuropathy.
Full article
►▼
Show Figures
Open AccessReview
The Feasibility and Applicability of Stem Cell Therapy for the Cure of Type 1 Diabetes
by
Ryota Inoue, Kuniyuki Nishiyama, Jinghe Li, Daisuke Miyashita, Masato Ono, Yasuo Terauchi and Jun Shirakawa
Cited by 6 | Viewed by 4166
Abstract
Stem cell therapy using islet-like insulin-producing cells derived from human pluripotent stem cells has the potential to allow patients with type 1 diabetes to withdraw from insulin therapy. However, several issues exist regarding the use of stem cell therapy to treat type 1
[...] Read more.
Stem cell therapy using islet-like insulin-producing cells derived from human pluripotent stem cells has the potential to allow patients with type 1 diabetes to withdraw from insulin therapy. However, several issues exist regarding the use of stem cell therapy to treat type 1 diabetes. In this review, we will focus on the following topics: (1) autoimmune responses during the autologous transplantation of stem cell-derived islet cells, (2) a comparison of stem cell therapy with insulin injection therapy, (3) the impact of the islet microenvironment on stem cell-derived islet cells, and (4) the cost-effectiveness of stem cell-derived islet cell transplantation. Based on these various viewpoints, we will discuss what is required to perform stem cell therapy for patients with type 1 diabetes.
Full article
►▼
Show Figures
Open AccessReview
Inducible Pluripotent Stem Cells as a Potential Cure for Diabetes
by
Kevin Verhoeff, Sarah J. Henschke, Braulio A. Marfil-Garza, Nidheesh Dadheech and Andrew Mark James Shapiro
Cited by 21 | Viewed by 6618
Abstract
Over the last century, diabetes has been treated with subcutaneous insulin, a discovery that enabled patients to forego death from hyperglycemia. Despite novel insulin formulations, patients with diabetes continue to suffer morbidity and mortality with unsustainable costs to the health care system. Continuous
[...] Read more.
Over the last century, diabetes has been treated with subcutaneous insulin, a discovery that enabled patients to forego death from hyperglycemia. Despite novel insulin formulations, patients with diabetes continue to suffer morbidity and mortality with unsustainable costs to the health care system. Continuous glucose monitoring, wearable insulin pumps, and closed-loop artificial pancreas systems represent an advance, but still fail to recreate physiologic euglycemia and are not universally available. Islet cell transplantation has evolved into a successful modality for treating a subset of patients with ‘brittle’ diabetes but is limited by organ donor supply and immunosuppression requirements. A novel approach involves generating autologous or immune-protected islet cells for transplant from inducible pluripotent stem cells to eliminate detrimental immune responses and organ supply limitations. In this review, we briefly discuss novel mechanisms for subcutaneous insulin delivery and define their shortfalls. We describe embryological development and physiology of islets to better understand their role in glycemic control and, finally, discuss cell-based therapies for diabetes and barriers to widespread use. In response to these barriers, we present the promise of stem cell therapy, and review the current gaps requiring solutions to enable widespread use of stem cells as a potential cure for diabetes.
Full article
►▼
Show Figures
Open AccessArticle
Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells
by
Jonathan Ribot, Cyprien Denoeud, Guilhem Frescaline, Rebecca Landon, Hervé Petite, Graciela Pavon-Djavid, Morad Bensidhoum and Fani Anagnostou
Cited by 5 | Viewed by 2588
Abstract
Bone marrow-derived multipotent stromal cells (BMMSCs) represent an attractive therapeutic modality for cell therapy in type 2 diabetes mellitus (T2DM)-associated complications. T2DM changes the bone marrow environment; however, its effects on BMMSC properties remain unclear. The present study aimed at investigating select functions
[...] Read more.
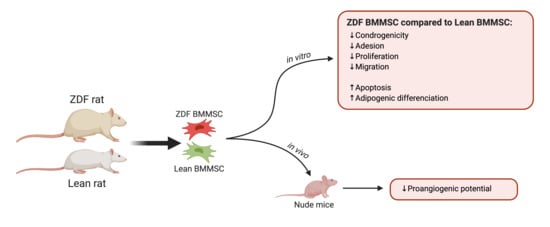
Bone marrow-derived multipotent stromal cells (BMMSCs) represent an attractive therapeutic modality for cell therapy in type 2 diabetes mellitus (T2DM)-associated complications. T2DM changes the bone marrow environment; however, its effects on BMMSC properties remain unclear. The present study aimed at investigating select functions and differentiation of BMMSCs harvested from the T2DM microenvironment as potential candidates for regenerative medicine. BMMSCs were obtained from Zucker diabetic fatty (ZDF; an obese-T2DM model) rats and their lean littermates (ZL; controls), and cultured under normoglycemic conditions. The BMMSCs derived from ZDF animals were fewer in number, with limited clonogenicity (by 2-fold), adhesion (by 2.9-fold), proliferation (by 50%), migration capability (by 25%), and increased apoptosis rate (by 2.5-fold) compared to their ZL counterparts. Compared to the cultured ZL-BMMSCs, the ZDF-BMMSCs exhibited (i) enhanced adipogenic differentiation (increased number of lipid droplets by 2-fold; upregulation of the Pparg, AdipoQ, and Fabp genes), possibly due to having been primed to undergo such differentiation in vivo prior to cell isolation, and (ii) different angiogenesis-related gene expression in vitro and decreased proangiogenic potential after transplantation in nude mice. These results provided evidence that the T2DM environment impairs BMMSC expansion and select functions pertinent to their efficacy when used in autologous cell therapies.
Full article
►▼
Show Figures
Open AccessArticle
Secreted Factors from Stem Cells of Human Exfoliated Deciduous Teeth Directly Activate Endothelial Cells to Promote All Processes of Angiogenesis
by
Makoto Kato, Shin Tsunekawa, Nobuhisa Nakamura, Emiri Miura-Yura, Yuichiro Yamada, Yusuke Hayashi, Hiromi Nakai-Shimoda, Saeko Asano, Tomohide Hayami, Mikio Motegi, Emi Asano-Hayami, Sachiko Sasajima, Yoshiaki Morishita, Tatsuhito Himeno, Masaki Kondo, Yoshiro Kato, Takako Izumoto-Akita, Akihito Yamamoto, Keiko Naruse, Jiro Nakamura and Hideki Kamiyaadd
Show full author list
remove
Hide full author list
| Viewed by 2689
Abstract
Diabetes is a major risk factor for atherosclerosis and ischemic vascular diseases. Recently, regenerative medicine is expected to be a novel therapy for ischemic diseases. Our previous studies have reported that transplantation of stem cells promoted therapeutic angiogenesis for diabetic neuropathy and ischemic
[...] Read more.
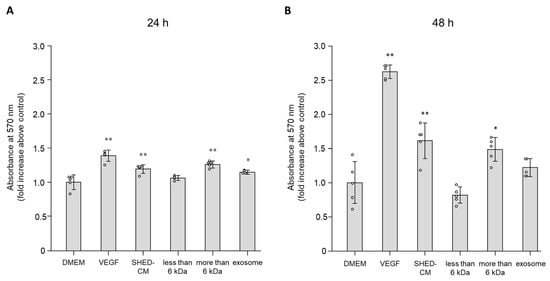
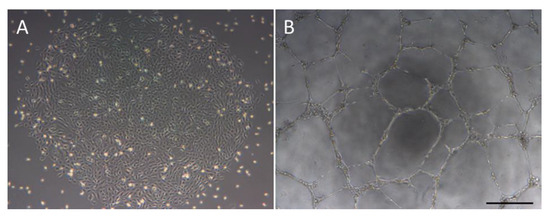
Diabetes is a major risk factor for atherosclerosis and ischemic vascular diseases. Recently, regenerative medicine is expected to be a novel therapy for ischemic diseases. Our previous studies have reported that transplantation of stem cells promoted therapeutic angiogenesis for diabetic neuropathy and ischemic vascular disease in a paracrine manner, but the precise mechanism is unclear. Therefore, we examined whether secreted factors from stem cells had direct beneficial effects on endothelial cells to promote angiogenesis. The soluble factors were collected as conditioned medium (CM) 48 h after culturing stem cells from human exfoliated deciduous teeth (SHED) in serum-free DMEM. SHED-CM significantly increased cell viability of human umbilical vein endothelial cells (HUVECs) in MTT assays and accelerated HUVECs migration in wound healing and Boyden chamber assays. In a Matrigel plug assay of mice, the migrated number of primary endothelial cells was markedly increased in the plug containing SHED-CM or SHED suspension. SHED-CM induced complex tubular structures of HUVECs in a tube formation assay. Furthermore, SHED-CM significantly increased neovascularization from the primary rat aorta, indicating that SHED-CM stimulated primary endothelial cells to promote comprehensive angiogenesis processes. The angiogenic effects of SHED-CM were the same or greater than the effective concentration of VEGF. In conclusion, SHED-CM directly stimulates vascular endothelial cells to promote angiogenesis and is promising for future clinical application.
Full article
►▼
Show Figures
Open AccessReview
The Functionality of Endothelial-Colony-Forming Cells from Patients with Diabetes Mellitus
by
Caomhán J. Lyons and Timothy O'Brien
Cited by 15 | Viewed by 3942
Abstract
Endothelial-colony-forming cells (ECFCs) are a population of progenitor cells which have demonstrated promising angiogenic potential both in vitro and in vivo. However, ECFCs from diabetic patients have been shown to be dysfunctional compared to ECFCs from healthy donors. Diabetes mellitus itself presents with
[...] Read more.
Endothelial-colony-forming cells (ECFCs) are a population of progenitor cells which have demonstrated promising angiogenic potential both in vitro and in vivo. However, ECFCs from diabetic patients have been shown to be dysfunctional compared to ECFCs from healthy donors. Diabetes mellitus itself presents with many vascular co-morbidities and it has been hypothesized that ECFCs may be a potential cell therapy option to promote revascularisation in these disorders. While an allogeneic cell therapy approach would offer the potential of an ‘off the shelf’ therapeutic product, to date little research has been carried out on umbilical cord-ECFCs in diabetic models. Alternatively, autologous cell therapy using peripheral blood-ECFCs allows the development of a personalised therapeutic approach to medicine; however, autologous diabetic ECFCs are dysfunctional and need to be repaired so they can effectively treat diabetic co-morbidities. Many different groups have modified autologous diabetic ECFCs to improve their function using a variety of methods including pre-treatment with different factors or with genetic modification. While the in vitro and in vivo data from the literature is promising, no ECFC therapy has proceeded to clinical trials to date, indicating that more research is needed for a potential ECFC therapy in the future to treat diabetic complications.
Full article
►▼
Show Figures
Open AccessReview
Identifying the Therapeutic Significance of Mesenchymal Stem Cells
by
Vineet Kumar Mishra, Hui-Hsuan Shih, Farzana Parveen, David Lenzen, Etsuro Ito, Te-Fu Chan and Liang-Yin Ke
Cited by 72 | Viewed by 7763
Abstract
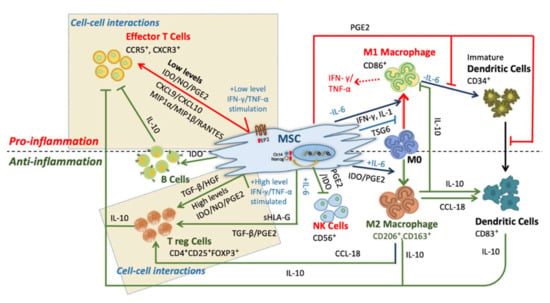
The pleiotropic behavior of mesenchymal stem cells (MSCs) has gained global attention due to their immense potential for immunosuppression and their therapeutic role in immune disorders. MSCs migrate towards inflamed microenvironments, produce anti-inflammatory cytokines and conceal themselves from the innate immune system. These
[...] Read more.
The pleiotropic behavior of mesenchymal stem cells (MSCs) has gained global attention due to their immense potential for immunosuppression and their therapeutic role in immune disorders. MSCs migrate towards inflamed microenvironments, produce anti-inflammatory cytokines and conceal themselves from the innate immune system. These signatures are the reason for the uprising in the sciences of cellular therapy in the last decades. Irrespective of their therapeutic role in immune disorders, some factors limit beneficial effects such as inconsistency of cell characteristics, erratic protocols, deviating dosages, and diverse transfusion patterns. Conclusive protocols for cell culture, differentiation, expansion, and cryopreservation of MSCs are of the utmost importance for a better understanding of MSCs in therapeutic applications. In this review, we address the immunomodulatory properties and immunosuppressive actions of MSCs. Also, we sum up the results of the enhancement, utilization, and therapeutic responses of MSCs in treating inflammatory diseases, metabolic disorders and diabetes.
Full article
►▼
Show Figures