Signaling Pathways in Cell Generation and Reprogramming
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 47519
Share This Topical Collection
Editor
 Dr. Kunimasa Ohta
Dr. Kunimasa Ohta
 Dr. Kunimasa Ohta
Dr. Kunimasa Ohta
E-Mail
Website
Collection Editor
Faculty of Arts and Science, Kyushu University, Fukuoka, Japan
Interests: reprogramming; transdifferentiation; multipotency; stem cell; somatic cell; lactic acid bacteria; ribosome
Topical Collection Information
Dear Colleagues,
Waddington’s epigenetic concept is widely supported as the cause of hierarchical restrictions and robustness of cell differentiation during normal development. However, pluripotency can be achieved artificially by transplanting the nuclei of frog somatic cells into eggs, suggesting the presence of molecule(s) that reprogram the intrinsic epigenetic information of the egg.
In this Topical Collection, the focus will be on the creation of multipotent cells using extrinsic materials. To date, generated using several different kinds of methods, including forced expression of specific transcription factors, incorporating ribosomes into fibroblasts, infection of leprosy bacilli into adult Schwann cells, and applying a cocktail of small-molecule compounds or cell-penetrating peptides to fibroblasts. Though these cells have both advantages or disadvantages regarding their use in either basic research or clinical application, we realize that even terminally differentiated cells possess the potential to be reprogrammed by extrinsic materials. Therefore, precise knowledge of the specific cellular and molecular signaling behind such reprogramming is a priority of the scientific community.
The aim of this Topical Collection is to provide an overview of the signaling pathways and molecules involved in the process of cell generation and reprogramming by extrinsic materials.
Dr. Kunimasa Ohta
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- reprogramming
- transdifferentiation
- creation
- multipotency
- stem cell
- somatic cell
- embryonic stem cell
- induced pluripotent stem (iPS) cells
- Muse cells
Published Papers (9 papers)
Open AccessReview
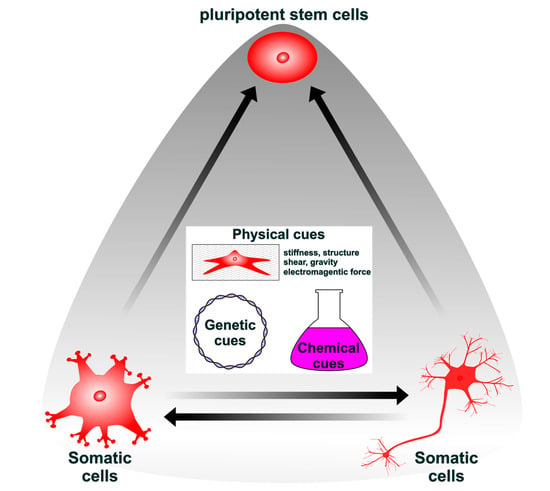
Lineage Reprogramming: Genetic, Chemical, and Physical Cues for Cell Fate Conversion with a Focus on Neuronal Direct Reprogramming and Pluripotency Reprogramming
by
Taichi Umeyama, Taito Matsuda and Kinichi Nakashima
Viewed by 394
Abstract
Although lineage reprogramming from one cell type to another is becoming a breakthrough technology for cell-based therapy, several limitations remain to be overcome, including the low conversion efficiency and subtype specificity. To address these, many studies have been conducted using genetics, chemistry, physics,
[...] Read more.
Although lineage reprogramming from one cell type to another is becoming a breakthrough technology for cell-based therapy, several limitations remain to be overcome, including the low conversion efficiency and subtype specificity. To address these, many studies have been conducted using genetics, chemistry, physics, and cell biology to control transcriptional networks, signaling cascades, and epigenetic modifications during reprogramming. Here, we summarize recent advances in cellular reprogramming and discuss future directions.
Full article
►▼
Show Figures
Open AccessArticle
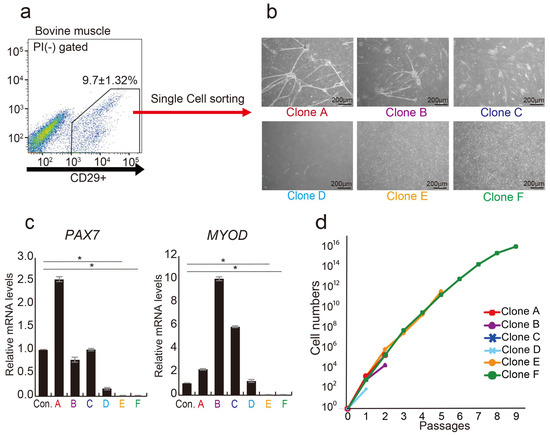
Quality Control of Stem Cell-Based Cultured Meat According to Specific Differentiation Abilities
by
Yuna Naraoka, Yo Mabuchi, Mai Kiuchi, Kyoko Kumagai, Daisuke Hisamatsu, Yosuke Yoneyama, Takanori Takebe and Chihiro Akazawa
Cited by 1 | Viewed by 1724
Abstract
The demand for stem cell-based cultured meat as an alternative protein source is increasing in response to global food scarcity. However, the definition of quality controls, including appropriate growth factors and cell characteristics, remains incomplete. Cluster of differentiation (CD) 29 is ubiquitously expressed
[...] Read more.
The demand for stem cell-based cultured meat as an alternative protein source is increasing in response to global food scarcity. However, the definition of quality controls, including appropriate growth factors and cell characteristics, remains incomplete. Cluster of differentiation (CD) 29 is ubiquitously expressed in bovine muscle tissue and is a marker of progenitor cells in cultured meat. However, CD29+ cells are naturally heterogeneous, and this quality control issue must be resolved. In this study, the aim was to identify the subpopulation of the CD29+ cell population with potential utility in cultured meat production. The CD29+ cell population exhibited heterogeneity, discernible through the CD44 and CD344 markers. CD29+CD44−CD344− cells displayed the ability for long-term culture, demonstrating high adipogenic potential and substantial lipid droplet accumulation, even within 3D cultures. Conversely, CD29+CD44+ cells exhibited rapid proliferation but were not viable for prolonged culture. Using cells suitable for adipocyte and muscle differentiation, we successfully designed meat buds, especially those rich in fat. Collectively, the identification and comprehension of distinct cell populations within bovine tissues contribute to quality control predictions in meat production. They also aid in establishing a stable and reliable cultured meat production technique.
Full article
►▼
Show Figures
Open AccessArticle
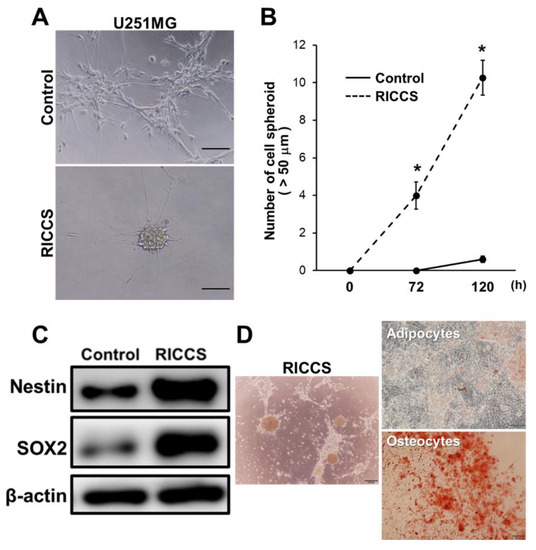
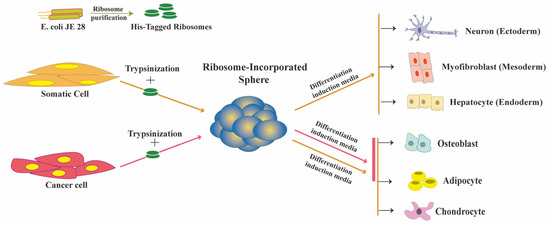
Glioma Cells Acquire Stem-like Characters by Extrinsic Ribosome Stimuli
by
Yuki Shirakawa, Kunimasa Ohta, Shunsuke Miyake, Ayumi Kanemaru, Akari Kuwano, Kou Yonemaru, Shota Uchino, Michiko Yamaoka, Yuki Ito, Naofumi Ito, Takuichiro Hide, Naoki Shinojima, Akitake Mukasa, Hideyuki Saito and Hirofumi Jono
Cited by 8 | Viewed by 3078
Abstract
Although glioblastoma (GBM) stem-like cells (GSCs), which retain chemo-radio resistance and recurrence, are key prognostic factors in GBM patients, the molecular mechanisms of GSC development are largely unknown. Recently, several studies revealed that extrinsic ribosome incorporation into somatic cells resulted in stem cell
[...] Read more.
Although glioblastoma (GBM) stem-like cells (GSCs), which retain chemo-radio resistance and recurrence, are key prognostic factors in GBM patients, the molecular mechanisms of GSC development are largely unknown. Recently, several studies revealed that extrinsic ribosome incorporation into somatic cells resulted in stem cell properties and served as a key trigger and factor for the cell reprogramming process. In this study, we aimed to investigate the mechanisms underlying GSCs development by focusing on extrinsic ribosome incorporation into GBM cells. Ribosome-induced cancer cell spheroid (RICCS) formation was significantly upregulated by ribosome incorporation. RICCS showed the stem-like cell characters (number of cell spheroid, stem cell markers, and ability for trans differentiation towards adipocytes and osteocytes). In RICCS, the phosphorylation and protein expression of ribosomal protein S6 (RPS6), an intrinsic ribosomal protein, and STAT3 phosphorylation were upregulated, and involved in the regulation of cell spheroid formation. Consistent with those results, glioma-derived extrinsic ribosome also promoted GBM-RICCS formation through intrinsic RPS6 phosphorylation. Moreover, in glioma patients, RPS6 phosphorylation was dominantly observed in high-grade glioma tissues, and predominantly upregulated in GSCs niches, such as the perinecrosis niche and perivascular niche. Those results indicate the potential biological and clinical significance of extrinsic ribosomal proteins in GSC development.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
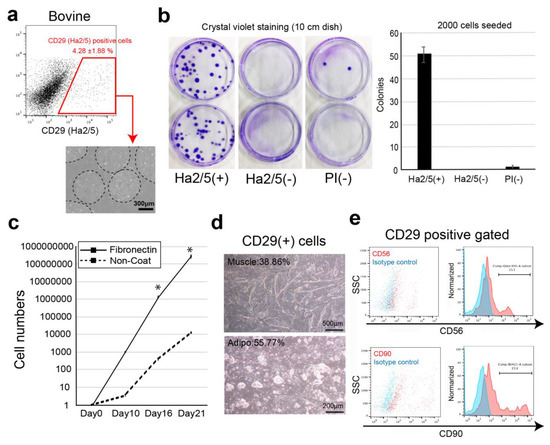
Isolation and Characterization of Tissue Resident CD29-Positive Progenitor Cells in Livestock to Generate a Three-Dimensional Meat Bud
by
Yuna Naraoka, Yo Mabuchi, Yosuke Yoneyama, Eriko Grace Suto, Daisuke Hisamatsu, Mami Ikeda, Risa Ito, Tetsuya Nakamura, Takanori Takebe and Chihiro Akazawa
Cited by 8 | Viewed by 5458
Abstract
The current process of meat production using livestock has significant effects on the global environment, including high emissions of greenhouse gases. In recent years, cultured meat has attracted attention as a way to acquire animal proteins. However, the lack of markers that isolate
[...] Read more.
The current process of meat production using livestock has significant effects on the global environment, including high emissions of greenhouse gases. In recent years, cultured meat has attracted attention as a way to acquire animal proteins. However, the lack of markers that isolate proliferating cells from bovine tissues and the complex structure of the meat make it difficult to culture meat in a dish. In this study, we screened 246 cell-surface antibodies by fluorescence-activated cell sorting for their capacity to form colonies and their suitability to construct spheroid “meat buds”. CD29+ cells (Ha2/5 clone) have a high potency to form colonies and efficiently proliferate on fibronectin-coated dishes. Furthermore, the meat buds created from CD29+ cells could differentiate into muscle and adipose cells in a three-dimensional structure. The meat buds embedded in the collagen gel proliferated in the matrix and formed large aggregates. Approximately 10 trillion cells can theoretically be obtained from 100 g of bovine tissue by culturing and amplifying them using these methods. The CD29+ cell characteristics of bovine tissue provide insights into the production of meat alternatives in vitro.
Full article
►▼
Show Figures
Open AccessReview
Ribosome-Induced Cellular Multipotency, an Emerging Avenue in Cell Fate Reversal
by
Arif Istiaq and Kunimasa Ohta
Cited by 3 | Viewed by 3999
Abstract
The ribosome, which is present in all three domains of life, plays a well-established, critical role in the translation process by decoding messenger RNA into protein. Ribosomal proteins, in contrast, appear to play non-translational roles in growth, differentiation, and disease. We recently discovered
[...] Read more.
The ribosome, which is present in all three domains of life, plays a well-established, critical role in the translation process by decoding messenger RNA into protein. Ribosomal proteins, in contrast, appear to play non-translational roles in growth, differentiation, and disease. We recently discovered that ribosomes are involved in reverting cellular potency to a multipotent state. Ribosomal incorporation (the uptake of free ribosome by living cells) can direct the fate of both somatic and cancer cells into multipotency, allowing them to switch cell lineage. During this process, both types of cells experienced cell-cycle arrest and cellular stress while remaining multipotent. This review provides a molecular perspective on current insights into ribosome-induced multipotency and sheds light on how a common stress-associated mechanism may be involved. We also discuss the impact of this phenomenon on cancer cell reprogramming and its potential in cancer therapy.
Full article
►▼
Show Figures
Open AccessArticle
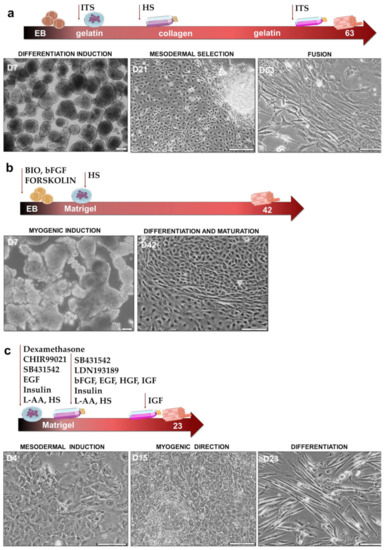
Myogenic Differentiation of iPS Cells Shows Different Efficiency in Simultaneous Comparison of Protocols
by
Aleksandra Ulman, Marta Kot, Klaudia Skrzypek, Barbara Szewczyk and Marcin Majka
Cited by 2 | Viewed by 4371
Abstract
Induced pluripotent stem (iPS) cells constitute a perfect tool to study human embryo development processes such as myogenesis, thanks to their ability to differentiate into three germ layers. Currently, many protocols to obtain myogenic cells have been described in the literature. They differ
[...] Read more.
Induced pluripotent stem (iPS) cells constitute a perfect tool to study human embryo development processes such as myogenesis, thanks to their ability to differentiate into three germ layers. Currently, many protocols to obtain myogenic cells have been described in the literature. They differ in many aspects, such as media components, including signaling modulators, feeder layer constituents, and duration of culture. In our study, we compared three different myogenic differentiation protocols to verify, side by side, their efficiency. Protocol I was based on embryonic bodies differentiation induction, ITS addition, and selection with adhesion to collagen I type. Protocol II was based on strong myogenic induction at the embryonic bodies step with BIO, forskolin, and bFGF, whereas cells in Protocol III were cultured in monolayers in three special media, leading to WNT activation and TGF-β and BMP signaling inhibition. Myogenic induction was confirmed by the hierarchical expression of myogenic regulatory factors MYF5, MYOD, MYF6 and MYOG, as well as the expression of myotubes markers MYH3 and MYH2, in each protocol. Our results revealed that Protocol III is the most efficient in obtaining myogenic cells. Furthermore, our results indicated that CD56 is not a specific marker for the evaluation of myogenic differentiation.
Full article
►▼
Show Figures
Open AccessReview
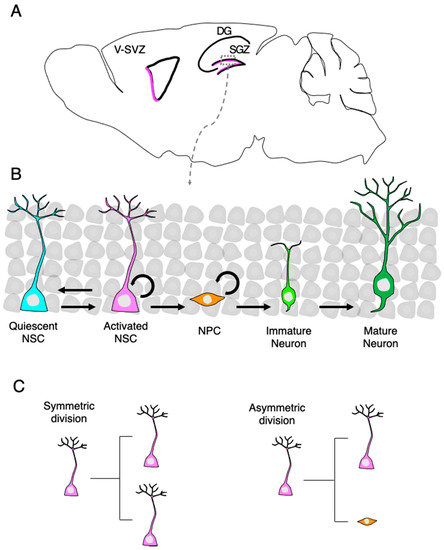
Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors
by
Shuzo Matsubara, Taito Matsuda and Kinichi Nakashima
Cited by 30 | Viewed by 4404
Abstract
Tissue-specific stem cells give rise to new functional cells to maintain tissue homeostasis and restore damaged tissue after injury. To ensure proper brain functions in the adult brain, neural stem cells (NSCs) continuously generate newborn neurons that integrate into pre-existing neuronal networks. Proliferation,
[...] Read more.
Tissue-specific stem cells give rise to new functional cells to maintain tissue homeostasis and restore damaged tissue after injury. To ensure proper brain functions in the adult brain, neural stem cells (NSCs) continuously generate newborn neurons that integrate into pre-existing neuronal networks. Proliferation, as well as neurogenesis of NSCs, are exquisitely controlled by extrinsic and intrinsic factors, and their underlying mechanisms have been extensively studied with the goal of enhancing the neurogenic capacity of NSCs for regenerative medicine. However, neurogenesis of endogenous NSCs alone is insufficient to completely repair brains damaged by neurodegenerative diseases and/or injury because neurogenic areas are limited and few neurons are produced in the adult brain. An innovative approach towards replacing damaged neurons is to induce conversion of non-neuronal cells residing in injured sites into neurons by a process referred to as direct reprogramming. This review describes extrinsic and intrinsic factors controlling NSCs and neurogenesis in the adult brain and discusses prospects for their applications. It also describes direct neuronal reprogramming technology holding promise for future clinical applications.
Full article
►▼
Show Figures
Open AccessReview
Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation
by
Chelsea Schiliro and Bonnie L. Firestein
Cited by 200 | Viewed by 18698
|
Correction
Abstract
Cancer cells alter metabolic processes to sustain their characteristic uncontrolled growth and proliferation. These metabolic alterations include (1) a shift from oxidative phosphorylation to aerobic glycolysis to support the increased need for ATP, (2) increased glutaminolysis for NADPH regeneration, (3) altered flux through
[...] Read more.
Cancer cells alter metabolic processes to sustain their characteristic uncontrolled growth and proliferation. These metabolic alterations include (1) a shift from oxidative phosphorylation to aerobic glycolysis to support the increased need for ATP, (2) increased glutaminolysis for NADPH regeneration, (3) altered flux through the pentose phosphate pathway and the tricarboxylic acid cycle for macromolecule generation, (4) increased lipid uptake, lipogenesis, and cholesterol synthesis, (5) upregulation of one-carbon metabolism for the production of ATP, NADH/NADPH, nucleotides, and glutathione, (6) altered amino acid metabolism, (7) metabolism-based regulation of apoptosis, and (8) the utilization of alternative substrates, such as lactate and acetate. Altered metabolic flux in cancer is controlled by tumor-host cell interactions, key oncogenes, tumor suppressors, and other regulatory molecules, including non-coding RNAs. Changes to metabolic pathways in cancer are dynamic, exhibit plasticity, and are often dependent on the type of tumor and the tumor microenvironment, leading in a shift of thought from the Warburg Effect and the “reverse Warburg Effect” to metabolic plasticity. Understanding the complex nature of altered flux through these multiple pathways in cancer cells can support the development of new therapies.
Full article
►▼
Show Figures
Open AccessArticle
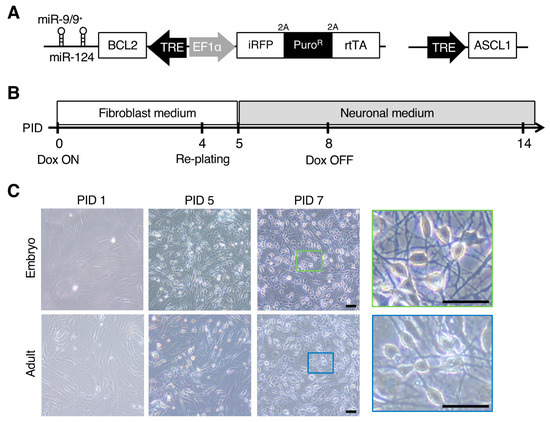
Direct Neuronal Reprogramming of Common Marmoset Fibroblasts by ASCL1, microRNA-9/9*, and microRNA-124 Overexpression
by
Akisa Nemoto, Reona Kobayashi, Sho Yoshimatsu, Yuta Sato, Takahiro Kondo, Andrew S. Yoo, Seiji Shiozawa and Hideyuki Okano
Cited by 5 | Viewed by 3438
Abstract
The common marmoset (
Callithrix jacchus) has attracted considerable attention, especially in the biomedical science and neuroscience research fields, because of its potential to recapitulate the complex and multidimensional phenotypes of human diseases, and several neurodegenerative transgenic models have been reported. However,
[...] Read more.
The common marmoset (
Callithrix jacchus) has attracted considerable attention, especially in the biomedical science and neuroscience research fields, because of its potential to recapitulate the complex and multidimensional phenotypes of human diseases, and several neurodegenerative transgenic models have been reported. However, there remain several issues as (i) it takes years to generate late-onset disease models, and (ii) the onset age and severity of phenotypes can vary among individuals due to differences in genetic background. In the present study, we established an efficient and rapid direct neuronal induction method (induced neurons; iNs) from embryonic and adult marmoset fibroblasts to investigate cellular-level phenotypes in the marmoset brain in vitro. We overexpressed reprogramming effectors, i.e., microRNA-9/9*, microRNA-124, and Achaete-Scute family bHLH transcription factor 1, in fibroblasts with a small molecule cocktail that facilitates neuronal induction. The resultant iNs from embryonic and adult marmoset fibroblasts showed neuronal characteristics within two weeks, including neuron-specific gene expression and spontaneous neuronal activity. As directly reprogrammed neurons have been shown to model neurodegenerative disorders, the neuronal reprogramming of marmoset fibroblasts may offer new tools for investigating neurological phenotypes associated with disease progression in non-human primate neurological disease models.
Full article
►▼
Show Figures