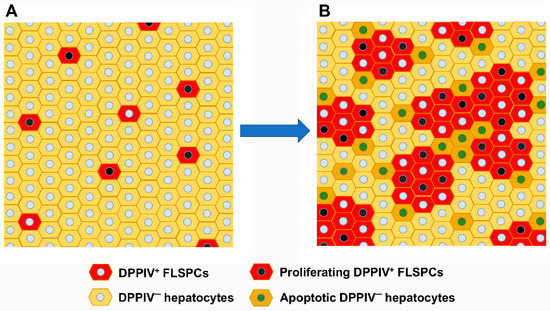
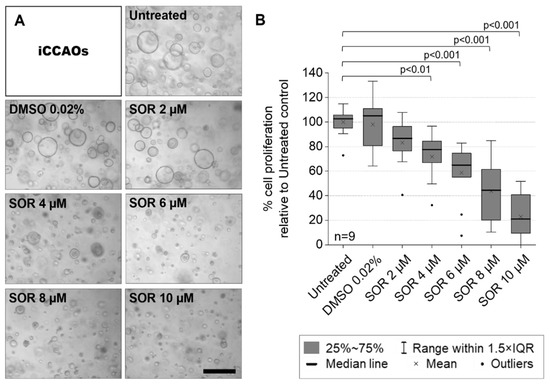
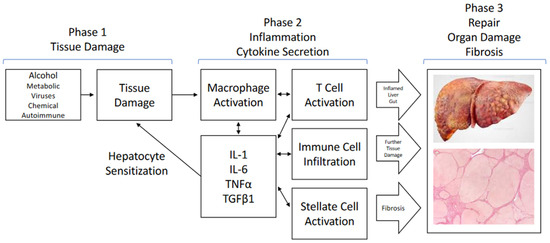
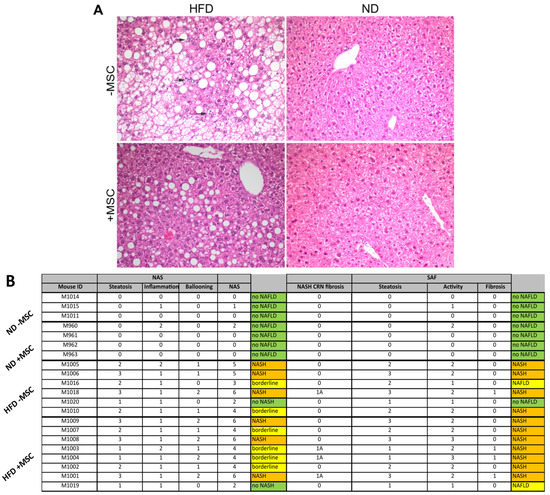
Progress in Liver Stem Cell Therapy
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 10357Editors
Interests: liver diseases; stem cell biology; regenerative medicine; stem cell differentiation; mesenchymal stem cell; stem cell isolation; liver metabolism; liver cell transplantation
Topical Collection Information
Dear Colleagues,
The transplantation of liver cells to treat liver diseases has a long history. As early as the mid-1970s, donor hepatocytes isolated from rats were transplanted via the portal vein into a recipient rat liver suffering from a defect of the bilirubin-conjugating enzyme uridine diphosphate glucuronosyltransferase. The defect was corrected at least in part for a sustained period of time after transplantation (AJ Matas et al., Science 1976;192:892-4). However, the clinical translation of the method is still awaiting breakthrough because of the rareness of donor livers for hepatocyte isolation. Since the feasibility of hepatocyte transplantation has, nevertheless, been proven in principle, alternative sources of transplantable hepatocytes have been explored. The most promising tools are stem cell-derived hepatocytes. These comprise hepatocyte-like cells derived from adult or embryonic stem cells and from induced pluripotent stem cells, besides others. Additionally, liver-like three-dimensional structures, named liver organoids, have been considered an adequate tissue replacement for diseased liver tissue. Recently, awareness has grown from mesenchymal stromal cells that, not the cells in toto, but even exosomes derived from them were sufficient to correct for liver tissue defects. Therefore, the scope of the Topical Collection “Progress in Liver Stem Cell Therapy” is to compile the foremost developments in stem cell-based approaches to treating liver diseases.
In the context of liver diseases, it is the aim to collect the latest research on novel stem cell resources and their pre-clinical as well as clinical establishment; animal and cell-culture model systems with which to assess stem cells’ therapeutic potential; concepts for the standardization of the isolation, processing and storage of stem cells and stem cell-based hepatocytes; and how stem cells impact liver diseases mechanistically. Contributions including bioinformatics and computational modeling concepts for predicting stem cell-based mechanisms and therapy outcomes are highly appreciated. We invite authors to submit their highly innovative research or comprehensive review articles related to the current and future development of the treatment of liver diseases with stem cell-based approaches.
Prof. Dr. Bruno Christ
Prof. Dr. Michael Oertel
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- liver
- hepatocyte
- stem cell
- cell transplantation
- organoids
- liver disease
- cell therapy
- experimental models
- transdifferentiation