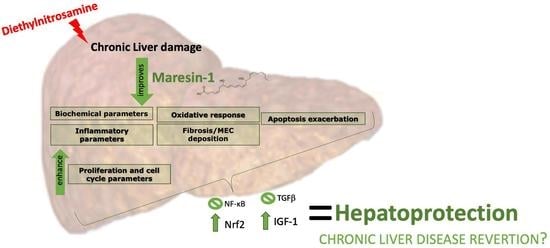
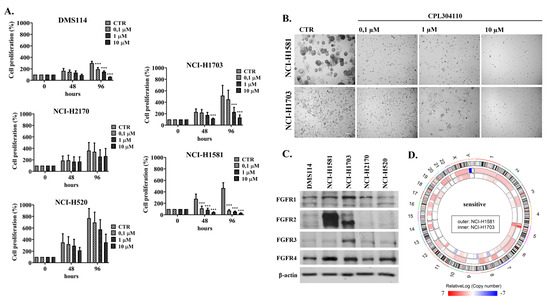
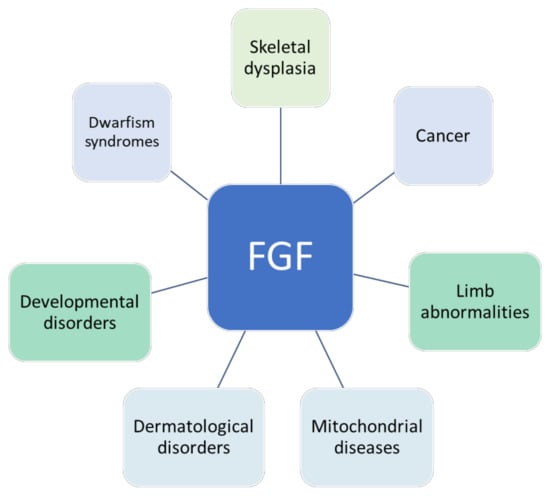
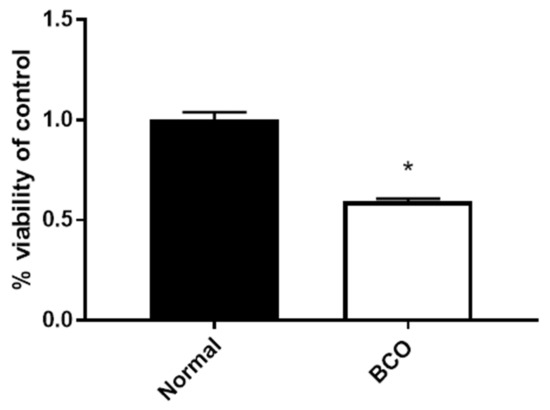
Fibroblast Growth Factors: Pathophysiology and Therapeutics
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 53100Editor
Interests: cytokines; cell signaling; design of novel fibroblast growth factor (FGF) variants; FGF-receptor activation, non-classical secretion of FGF1; FGF pathogenesis; drug discovery
Topical Collection Information
Dear Colleagues,
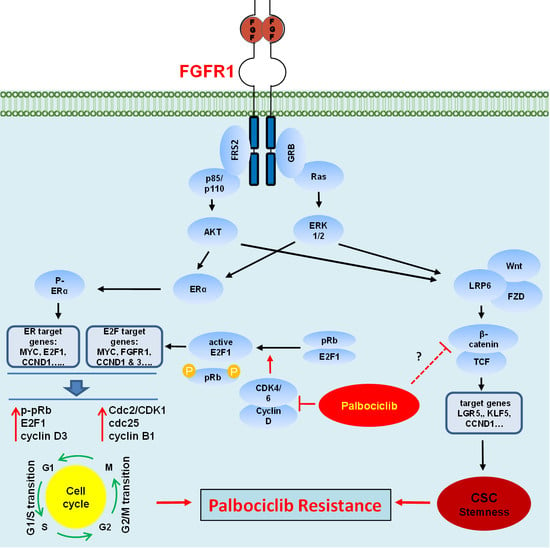
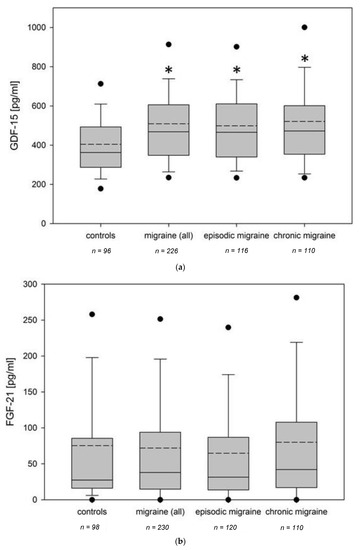
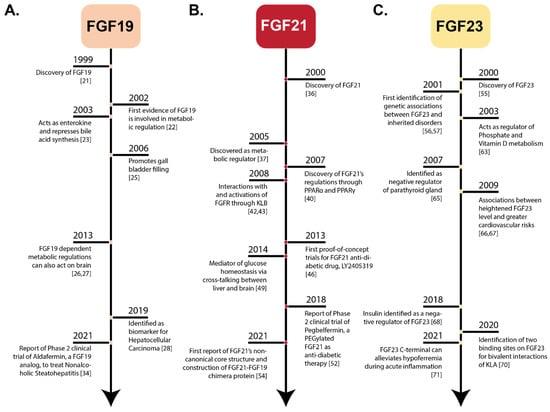
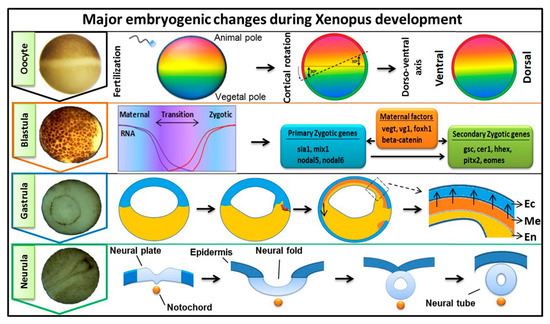
Fibroblast growth factors (FGFs) are a family of cytokines that play crucial roles in the regulation of key cellular processes such as cell proliferation, cell differentiation, tissue morphogenesis, angiogenesis, wound repair, and tumor growth. Members of the FGF family, with the exception of FGF11, FGF12, FGF13, and FGF14, elicit a cellular response by specifically binding to their cell-surface tyrosine kinase receptors and consequently activate a cascade of intracellular signaling pathways, including the PI3 kinase/AKT p, RAS/MAP kinase, and PLCγ pathways. Most members of the FGF family that bind to heparin sulfate are paracrine secretors. However, FGF19, FGF21, and FGF23 function as endocrine secretors, recruit the Klotho proteins, and activate the FGF receptors in a heparin-independent manner. These endocrine FGFs have been demonstrated to be critical regulators of glucose and lipid metabolism, vitamin D biosynthesis, and phosphate homeostasis and, hence, have attracted an immense amount of interest as therapeutics against diabetes, obesity, osteoporosis, cardiovascular and kidney diseases, and a wide array of diseases associated with the liver–gut axis. Additionally, aberrations in the FGF signaling process are the cause of many skeletal syndromes, including Kallmann syndrome, Lacrimo-auriculo-dento-digital syndrome (LADD syndrome), and cancer. Recent determination of the three-dimensional structures of the FGF signaling complex has provided us with valuable new insights into the molecular bases of a multitude of types of FGF-mediated pathogenesis. In addition, the availability of structural information on the structure–activity relationship of FGFs has opened new avenues to the rational design of drugs against FGF-mediated pathogenesis. In this context, this Topical Collection of Cells provides an exciting open-access platform for original research articles, shorter perspective articles, and comprehensive reviews on significant recent advances in FGF pathophysiology and therapeutics against the plethora of types of FGF-related pathogenesis.
Prof. T.K.S. Kumar
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cytokines
- structure and function of FGFs
- FGF signaling and mitogenic activity
- angiogensis and cardiovascular protection
- craniofacial syndromes
- FGF chimeras
- parcrine and endocrine secretors
- glucose and phosphate metabolism
- wound healing
- stem cells and tissue regenration
- therapeutics against FGFs