Regulation of Eukaryotic Cytochrome c Oxidase
A topical collection in Cells (ISSN 2073-4409).
Viewed by 35072Editors
Interests: respiration; mitochondria; cytochrome c oxidase; calcium signaling
2. Department of Heart Surgery, The University Hospital of Giessen and Marburg, Location Marburg, Baldinger Strasse 1, D-35043 Marburg, Germany
Interests: physiological and pathophysiological role of mitochondria in the heart; function and dysfunction of cytochrome c oxidase; pharmaco-bioenergetics; structural and functional organization of mitochondrial respiratory chain
2. Department of Heart Surgery, The University Hospital of Giessen and Marburg, Location Marburg, Baldinger Strasse 1, D-35043 Marburg, Germany
Interests: metabolism in the ischemic heart; mitochondrial respiration; cytochrome c oxidase in stress; myocardprotection during heart surgery; pharmaco-bioenergetics
Topical Collection Information
Dear Colleagues,
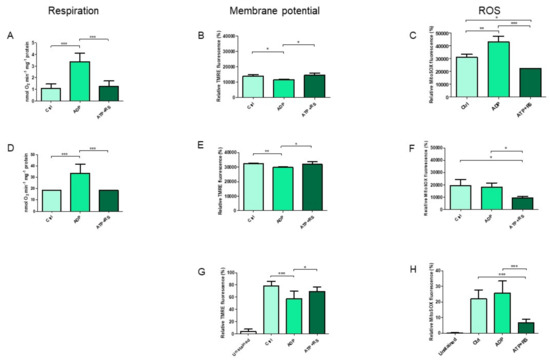
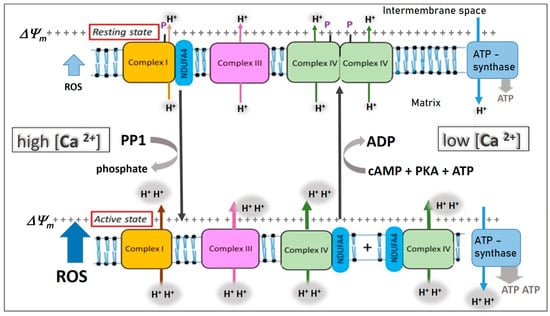
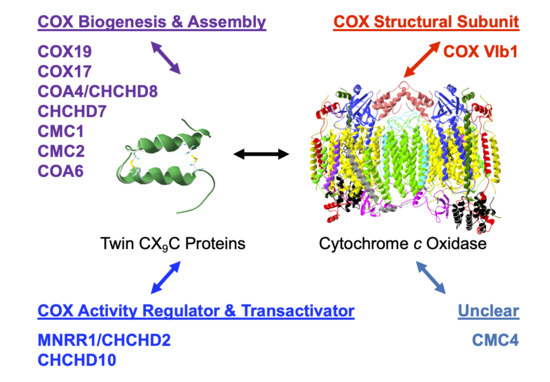
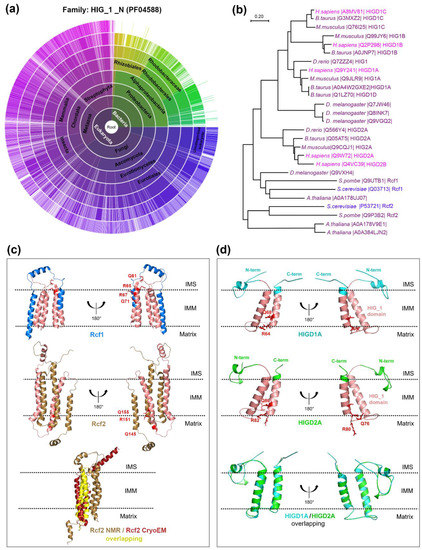
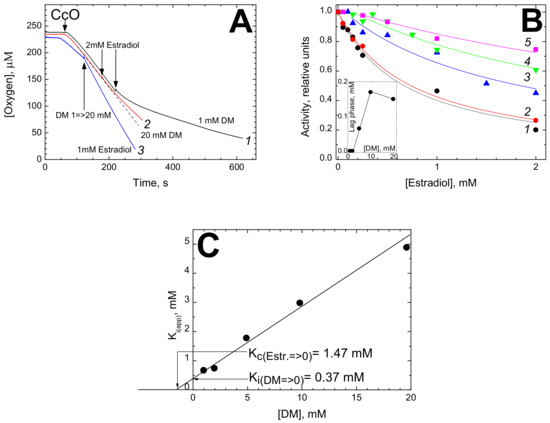
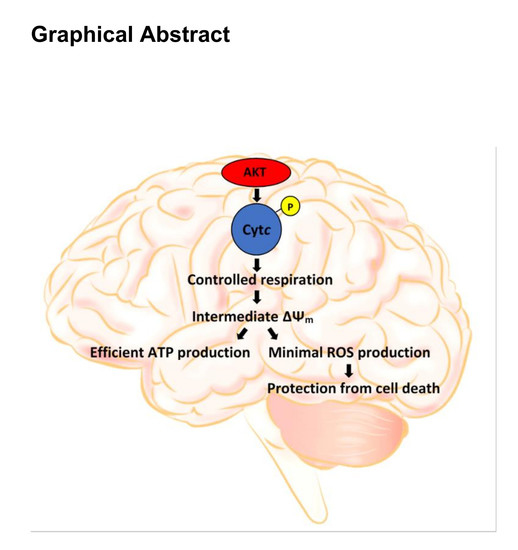
A Topical Collection on “Regulation of Eucaryotic Cytochrome c oxidase” is planned to be published by the journal “Cells” this year. In this issue we intend to compile papers on the regulation of eucaryotic cytochrome c oxidase (CytOx) and mitochondrial respiration. Hence, we prefer to include papers presenting the regulation of CytOx activity having physiological significance. The detailed mechanisms of electron and proton transfer within the mitochondrially-encoded catalytic subunits I–III, which are similar to bacterial CytOx subunits I–III, will not be covered. Data on the regulation of CytOx activity by interaction with low molecular weight compounds (e.g. adenine nucleotides, calcium, zinc, di-iodothyronine) and with proteins (r.g., NDUFA4, Higda-1), regulation by expression of subunit isozymes, by phosphorylation and other chemical modifications, by supercomplex formation (e.g. respirasome) are welcome. In addition influence of subunit overexpression during cancerogenesis are wellcome.
Prof. Dr. Bernhard Kadenbach
Dr. Rabia Ramzan
Prof. Dr. Sebastian Vogt
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- phosphorylation of CytOx
- regulation of mitochondrial respiration
- expression of CytOx isozymes
- overexpression of CytOx subunits during cancerogenesis
- CytOx regulation in supercomplexes
- CytOx dimerization/monomerization
- allosteric ATP-inhibition of CytOx