Tumor Microenvironment: Interaction and Metabolism
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Metabolism".
Viewed by 5392Editors
Interests: tumor microenvironment; hypoxia; tumor immune response; natural killer; cytotoxic T lymphocytes; autophagy; cancer immunotherapy; immune checkpoint blockades; tumor immune landscape
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Following the successful publication of the first Special Issue of “Tumor Microenvironment: Interaction and Metabolism”, we are very pleased to launch the new edition, which is dedicated to highlighting recent developments in the field.
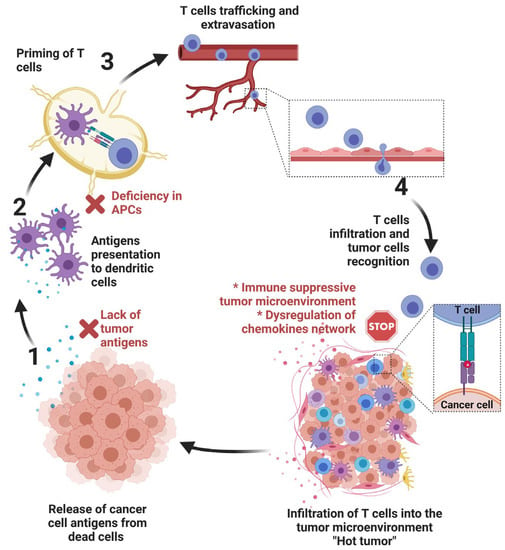
Within a tumor, cancer cells co-exist with other cells (including infiltrated immune cells, endothelial cells of the blood vessels, fibroblasts, and other stromal cells), which together form a niche for cancer development termed the “tumor microenvironment (TME)”. The TME is currently considered an integral part of the tumor organ and key element in shaping the tumor progression and its response to anticancer therapies. Normal cells in the TME are educated by cancer cells to support their own survival benefit through a complex array of interactions and coordinated crosstalks.
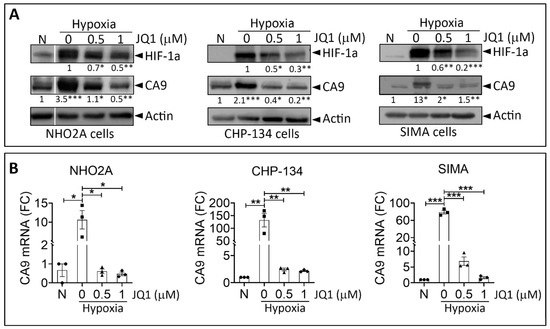
Significant progress has been made in our understanding of the different modes of communication within the TME and revealed potential therapeutic targets within the TME. In addition to different cell types, several hallmarks have been identified in the TME, including hypoxia, acidosis, and increased extracellular matrix stiffness. In such a hostile TME, tumor cells rewire their metabolic properties to shape hypoxic stress, resist anticancer therapies, and escape the immune system by turning the physiological roles of different constituents of the TME to ultimately promote tumor progression. Such cancer cell adaptations can be executed autonomously or through interactions with other cells in the tumor microenvironment.
In keeping with this, it has become clear that the TME is a sweeping landscape that should be considered for the development of more effective anticancer therapies based on combining agents targeting key factors in the TME. However, elaborating such combination therapies remains very challenging, since it relies on better knowledge of the interaction between cancer cells and their TME. This Special Issue is dedicated to providing an overview of the accumulating depth of knowledge about the role of the tumor microenvironment in cancer. A special focus is given on efforts undertaken to identify key druggable factors and pathways in the TME that can be manipulated to improve the efficacy of current cancer therapies and provide innovative drugs to the arsenals of cancer therapy.
Dr. Bassam Janji
Dr. Anwar Chammout
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- hypoxia
- tumor microenvironment
- cancer cell metabolism
- stromal cells
- tumor immune landscape
- cancer immunotherapy
- cancer metabolic switch
- tumor cell plasticity
- autophagy
- tumor vasculature