Role of Stem Cells in Spinal Cord Injuries
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 56066
Share This Topical Collection
Editors
 Dr. Kee D. Kim
Dr. Kee D. Kim
 Dr. Kee D. Kim
Dr. Kee D. Kim
E-Mail
Website
Collection Editor
Department of Neurological Surgery, University of California Davis, Sacramento, CA 95616, USA
Interests: spine clincial trials; biologics; stem cells; robotic spine surgery; regenerative medicine; artificial disc
 Dr. Julius O. Ebinu
Dr. Julius O. Ebinu
 Dr. Julius O. Ebinu
Dr. Julius O. Ebinu
E-Mail
Website
Collection Editor
Department of Neurological Surgery, University of California Davis, Sacramento, CA 95616, USA
Interests: stem cells; tissue bioengineering/3D printing; regenerative medicine; spinal cord injury; cell signaling; nanoparticles; brain–computer interface
Topical Collection Information
Dear Colleagues,
Spinal cord injury is a devastating complication of trauma that often results in various degrees of neurological compromise. Loss of motor and/or sensory function is a sequelae of both the primary spinal cord insult and a cascade of secondary events that include inflammation, neuronal cell death and formation of a glial scar. The pathophysiological events that occur after the primary spinal cord injury serve to create an environment that impairs axonal regrowth and recovery.
Various regenerative strategies to facilitate functional recovery after SCI have been explored with the goal of promoting axonal regeneration and myelination. Emerging trends utilizing cell replacement strategies have been pursued with the hopes of providing restorative targets to make new connections and/or remyelinate damaged axons. Several promising trials evaluating the effect of transplanting a variety of stem cells or stem cell-derived cells in various spinal cord injury models have yielded promising results. Moreover, the identification of the regenerative capacity of transplanted stem cells and their use in restorative therapies has led to an alternative approach of recruiting endogenous neural stem cells resident in the injured spinal cord.
This Topical Collection aims to summarize the current knowledge on the beneficial role of stem cells in neural regeneration and functional recovery in spinal cord injury.
Dr. Kee D. Kim
Dr. Julius O. Ebinu
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- stem cells
- spinal cord injury
- cell transplantation
- neurogenesis
Published Papers (14 papers)
Open AccessArticle
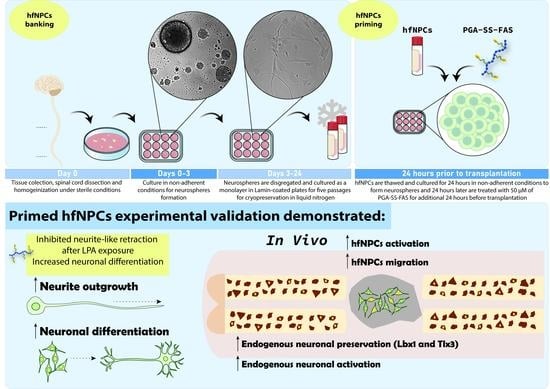
Transplantation of Human-Fetal-Spinal-Cord-Derived NPCs Primed with a Polyglutamate-Conjugated Rho/Rock Inhibitor in Acute Spinal Cord Injury
by
Esther Giraldo, Pablo Bonilla, Mara Mellado, Pablo Garcia-Manau, Carlota Rodo, Ana Alastrue, Eric Lopez, Elena Carreras Moratonas, Ferran Pellise, Snežana Đorđević, María J. Vicent and Victoria Moreno Manzano
Viewed by 1959
Abstract
Neural precursor cell (NPC) transplantation represents a promising therapy for treating spinal cord injuries (SCIs); however, despite successful results obtained in preclinical models, the clinical translation of this approach remains challenging due, in part, to the lack of consensus on an optimal cell
[...] Read more.
Neural precursor cell (NPC) transplantation represents a promising therapy for treating spinal cord injuries (SCIs); however, despite successful results obtained in preclinical models, the clinical translation of this approach remains challenging due, in part, to the lack of consensus on an optimal cell source for human neuronal cells. Depending on the cell source, additional limitations to NPC-based therapies include high tumorigenic potential, alongside poor graft survival and engraftment into host spinal tissue. We previously demonstrated that NPCs derived from rat fetal spinal cords primed with a polyglutamate (PGA)-conjugated form of the Rho/Rock inhibitor fasudil (PGA-SS-FAS) displayed enhanced neuronal differentiation and graft survival when compared to non-primed NPCs. We now conducted a similar study of human-fetal-spinal-cord-derived NPCs (hfNPCs) from legal gestational interruptions at the late gestational stage, at 19–21.6 weeks. In vitro, expanded hfNPCs retained neural features, multipotency, and self-renewal, which supported the development of a cell banking strategy. Before transplantation, we established a simple procedure to prime hfNPCs by overnight incubation with PGA-SS-FAS (at 50 μM FAS equiv.), which improved neuronal differentiation and overcame neurite-like retraction after lysophosphatidic-acid-induced Rho/Rock activation. The transplantation of primed hfNPCs into immune-deficient mice (NU(
NCr)-Foxn1
nu) immediately after the eighth thoracic segment compression prompted enhanced migration of grafted cells from the dorsal to the ventral spinal cord, increased preservation of GABAergic inhibitory Lbx1-expressing and glutamatergic excitatory Tlx3-expressing somatosensory interneurons, and elevated the numbers of preserved, c-Fos-expressing, activated neurons surrounding the injury epicenter, all in a low percentage. Overall, the priming procedure using PGA-SS-FAS could represent an alternative methodology to improve the capabilities of the hfNPC lines for a translational approach for acute SCI treatment.
Full article
►▼
Show Figures
Open AccessArticle
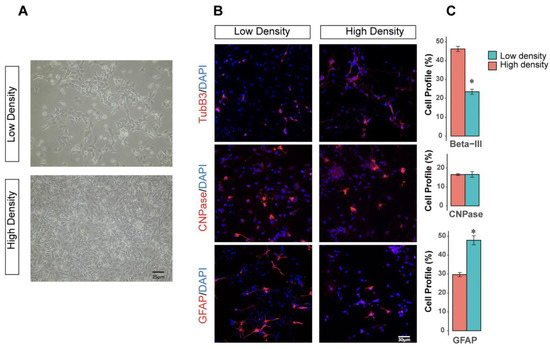
Cell–Cell Contact Mediates Gene Expression and Fate Choice of Human Neural Stem/Progenitor Cells
by
William B. McIntyre, Mehran Karimzadeh, Yasser Riazalhosseini, Mohamad Khazaei and Michael G. Fehlings
Cited by 2 | Viewed by 3756
Abstract
Transplantation of Neural Stem/Progenitor Cells (NPCs) is a promising regenerative strategy to promote neural repair following injury and degeneration because of the ability of these cells to proliferate, migrate, and integrate with the host tissue. Precise in vitro control of NPC proliferation without
[...] Read more.
Transplantation of Neural Stem/Progenitor Cells (NPCs) is a promising regenerative strategy to promote neural repair following injury and degeneration because of the ability of these cells to proliferate, migrate, and integrate with the host tissue. Precise in vitro control of NPC proliferation without compromising multipotency and differentiation ability is critical in stem cell maintenance. This idea was highlighted in recent clinical trials, where discrepancies in NPC culturing protocols produced inconsistent therapeutic benefits. Of note, cell density plays an important role in regulating the survival, proliferation, differentiation, and fate choice of stem cells. To determine the extent of variability produced by inconsistent culturing densities, the present study cultured human-induced pluripotent NPCs (hiPSC-NPCs) at either a low or high plating density. hiPSC-NPCs were then isolated for transcriptomic analysis or differentiation in vitro. Following sequencing analysis, genes involved in cell–cell contact-mediated pathways, including Hippo-signaling, NOTCH, and WNT were differentially expressed. Modulation of these pathways was highly associated with the regulation of pro-neuronal transcription factors, which were also upregulated in response to higher-density hiPSC-NPC culture. Moreover, higher plating density translated into a greater neuronal and less astrocytic differentiation in vitro. This study highlights the importance of precisely controlling culture conditions during the development of NPC transplantation therapies.
Full article
►▼
Show Figures
Open AccessReview
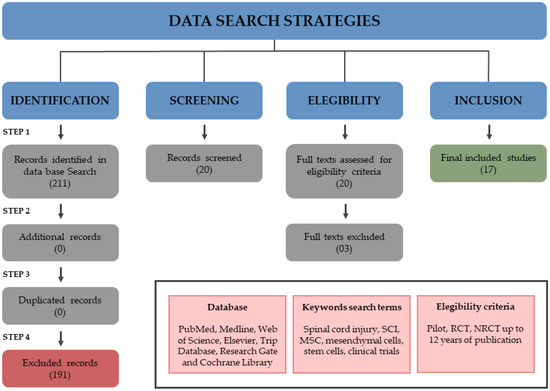
Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence
by
Lila Teixeira de Araújo, Carolina Thé Macêdo, Patrícia Kauanna Fonseca Damasceno, Ítalo Gabriel Costa das Neves, Carla Souza de Lima, Girlaine Café Santos, Thaís Alves de Santana, Gabriela Louise de Almeida Sampaio, Daniela Nascimento Silva, Cristiane Flora Villarreal, Alessandra Casemiro de Campos Chaguri, Crislaine Gomes da Silva, Augusto César de Andrade Mota, Roberto Badaró, Ricardo Ribeiro dos Santos and Milena Botelho Pereira Soares
Cited by 12 | Viewed by 3794
Abstract
Spinal cord injury (SCI) remains an important public health problem which often causes permanent loss of muscle strength, sensation, and function below the site of the injury, generating physical, psychological, and social impacts throughout the lives of the affected individuals, since there are
[...] Read more.
Spinal cord injury (SCI) remains an important public health problem which often causes permanent loss of muscle strength, sensation, and function below the site of the injury, generating physical, psychological, and social impacts throughout the lives of the affected individuals, since there are no effective treatments available. The use of stem cells has been investigated as a therapeutic approach for the treatment of SCI. Although a significant number of studies have been conducted in pre-clinical and clinical settings, so far there is no established cell therapy for the treatment of SCI. One aspect that makes it difficult to evaluate the efficacy is the heterogeneity of experimental designs in the clinical trials that have been published. Cell transplantation methods vary widely among the trials, and there are still no standardized protocols or recommendations for the therapeutic use of stem cells in SCI. Among the different cell types, mesenchymal stem/stromal cells (MSCs) are the most frequently tested in clinical trials for SCI treatment. This study reviews the clinical applications of MSCs for SCI, focusing on the critical analysis of 17 clinical trials published thus far, with emphasis on their design and quality. Moreover, it highlights the need for more evidence-based studies designed as randomized controlled trials and potential challenges to be addressed in context of stem cell therapies for SCI.
Full article
►▼
Show Figures
Open AccessReview
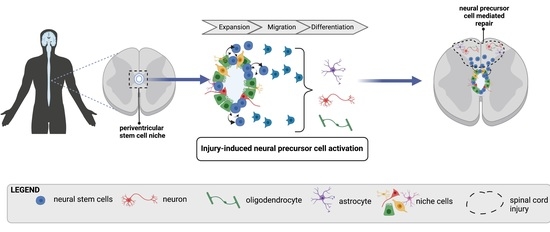
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair
by
Emily A. B. Gilbert, Nishanth Lakshman, Kylie S. K. Lau and Cindi M. Morshead
Cited by 25 | Viewed by 5842
Abstract
Spinal cord injury (SCI) affects millions of individuals worldwide. Currently, there is no cure, and treatment options to promote neural recovery are limited. An innovative approach to improve outcomes following SCI involves the recruitment of endogenous populations of neural stem cells (NSCs). NSCs
[...] Read more.
Spinal cord injury (SCI) affects millions of individuals worldwide. Currently, there is no cure, and treatment options to promote neural recovery are limited. An innovative approach to improve outcomes following SCI involves the recruitment of endogenous populations of neural stem cells (NSCs). NSCs can be isolated from the neuroaxis of the central nervous system (CNS), with brain and spinal cord populations sharing common characteristics (as well as regionally distinct phenotypes). Within the spinal cord, a number of NSC sub-populations have been identified which display unique protein expression profiles and proliferation kinetics. Collectively, the potential for NSCs to impact regenerative medicine strategies hinges on their cardinal properties, including self-renewal and multipotency (the ability to generate de novo neurons, astrocytes, and oligodendrocytes). Accordingly, endogenous NSCs could be harnessed to replace lost cells and promote structural repair following SCI. While studies exploring the efficacy of this approach continue to suggest its potential, many questions remain including those related to heterogeneity within the NSC pool, the interaction of NSCs with their environment, and the identification of factors that can enhance their response. We discuss the current state of knowledge regarding populations of endogenous spinal cord NSCs, their niche, and the factors that regulate their behavior. In an attempt to move towards the goal of enhancing neural repair, we highlight approaches that promote NSC activation following injury including the modulation of the microenvironment and parenchymal cells, pharmaceuticals, and applied electrical stimulation.
Full article
►▼
Show Figures
Open AccessArticle
The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro
by
Mohamed Tail, Hao Zhang, Guoli Zheng, Maryam Hatami, Thomas Skutella, Andreas Unterberg, Klaus Zweckberger and Alexander Younsi
Cited by 6 | Viewed by 2603
Abstract
The Sonic Hedgehog protein (Shh) has been extensively researched since its discovery in 1980. Its crucial role in early neurogenesis and endogenous stem cells of mature brains, as well as its recently described neuroprotective features, implicate further important effects on neuronal homeostasis. Here,
[...] Read more.
The Sonic Hedgehog protein (Shh) has been extensively researched since its discovery in 1980. Its crucial role in early neurogenesis and endogenous stem cells of mature brains, as well as its recently described neuroprotective features, implicate further important effects on neuronal homeostasis. Here, we investigate its potential role in the survival, proliferation, and differentiation of neural precursors cells (NPCs) under inflammatory stress as a potential adjunct for NPC-transplantation strategies in spinal cord injury (SCI) treatment. To this end, we simulated an inflammatory environment in vitro using lipopolysaccharide (LPS) and induced the Shh-pathway using recombinant Shh or blocked it using Cyclopamine, a potent Smo inhibitor. We found that Shh mediates the proliferation and neuronal differentiation potential of NPCs in vitro, even in an inflammatory stress environment mimicking the subacute phase after SCI. At the same time, our results indicate that a reduction of the Shh-pathway activation by blockage with Cyclopamine is associated with reduced NPC-survival, reduced neuronal differentiation and increased astroglial differentiation. Shh might thus, play a role in endogenous NPC-mediated neuroregeneration or even be a potent conjunct to NPC-based therapies in the inflammatory environment after SCI.
Full article
►▼
Show Figures
Open AccessReview
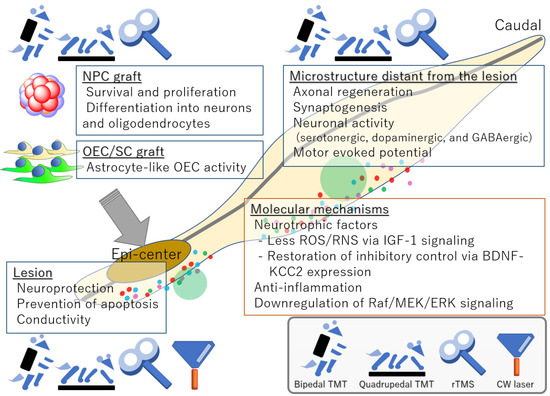
Regenerative Rehabilitation and Stem Cell Therapy Targeting Chronic Spinal Cord Injury: A Review of Preclinical Studies
by
Syoichi Tashiro, Masaya Nakamura and Hideyuki Okano
Cited by 17 | Viewed by 3379
Abstract
Stem cell medicine has led to functional recovery in the acute-to-subacute phase of spinal cord injury (SCI), but not yet in the chronic phase, during which various molecular mechanisms drastically remodel the tissue and render it treatment-resistant. Researchers are attempting to identify effective
[...] Read more.
Stem cell medicine has led to functional recovery in the acute-to-subacute phase of spinal cord injury (SCI), but not yet in the chronic phase, during which various molecular mechanisms drastically remodel the tissue and render it treatment-resistant. Researchers are attempting to identify effective combinatorial treatments that can overcome the refractory state of the chronically injured spinal cord. Regenerative rehabilitation, combinatorial treatment with regenerative medicine that aims to elicit synergistic effects, is being developed. Rehabilitation upon SCI in preclinical studies has recently attracted more attention because it is safe, induces neuronal plasticity involving transplanted stem cells and sensorimotor circuits, and is routinely implemented in human clinics. However, regenerative rehabilitation has not been extensively reviewed, and only a few reviews have focused on the use of physical medicine modalities for rehabilitative purposes, which might be more important in the chronic phase. Here, we summarize regenerative rehabilitation studies according to the effector, site, and mechanism. Specifically, we describe effects on transplanted cells, microstructures at and distant from the lesion, and molecular changes. To establish a treatment regimen that induces robust functional recovery upon chronic SCI, further investigations are required of combinatorial treatments incorporating stem cell therapy, regenerative rehabilitation, and medication.
Full article
►▼
Show Figures
Open AccessReview
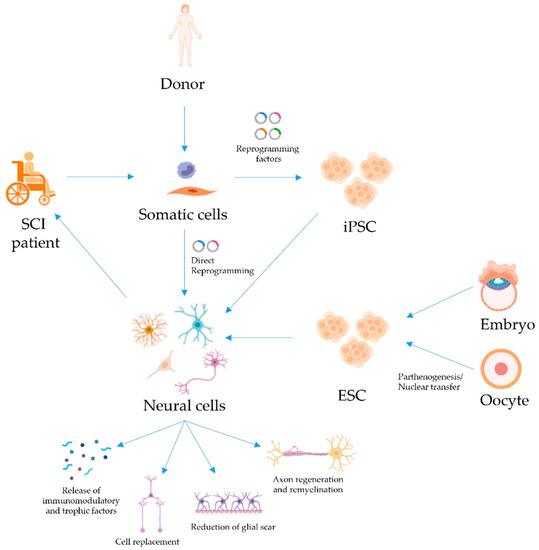
Pluripotent Stem Cells for Spinal Cord Injury Repair
by
Maria Martin-Lopez, Beatriz Fernandez-Muñoz and Sebastian Canovas
Cited by 6 | Viewed by 3351
Abstract
Spinal cord injury (SCI) is a devastating condition of the central nervous system that strongly reduces the patient’s quality of life and has large financial costs for the healthcare system. Cell therapy has shown considerable therapeutic potential for SCI treatment in different animal
[...] Read more.
Spinal cord injury (SCI) is a devastating condition of the central nervous system that strongly reduces the patient’s quality of life and has large financial costs for the healthcare system. Cell therapy has shown considerable therapeutic potential for SCI treatment in different animal models. Although many different cell types have been investigated with the goal of promoting repair and recovery from injury, stem cells appear to be the most promising. Here, we review the experimental approaches that have been carried out with pluripotent stem cells, a cell type that, due to its inherent plasticity, self-renewal, and differentiation potential, represents an attractive source for the development of new cell therapies for SCI. We will focus on several key observations that illustrate the potential of cell therapy for SCI, and we will attempt to draw some conclusions from the studies performed to date.
Full article
►▼
Show Figures
Open AccessArticle
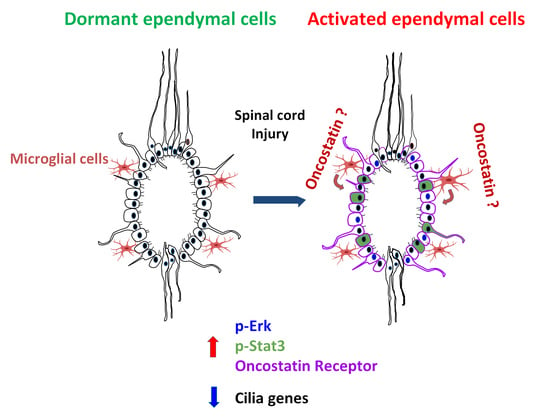
RNA Profiling of Mouse Ependymal Cells after Spinal Cord Injury Identifies the Oncostatin Pathway as a Potential Key Regulator of Spinal Cord Stem Cell Fate
by
Robert Chevreau, Hussein Ghazale, Chantal Ripoll, Chaima Chalfouh, Quentin Delarue, Anne Laure Hemonnot-Girard, Daria Mamaeva, Helene Hirbec, Bernard Rothhut, Shalaka Wahane, Florence Evelyne Perrin, Harun Najib Noristani, Nicolas Guerout and Jean Philippe Hugnot
Cited by 7 | Viewed by 4129
Abstract
Ependymal cells reside in the adult spinal cord and display stem cell properties in vitro. They proliferate after spinal cord injury and produce neurons in lower vertebrates but predominantly astrocytes in mammals. The mechanisms underlying this glial-biased differentiation remain ill-defined. We addressed this
[...] Read more.
Ependymal cells reside in the adult spinal cord and display stem cell properties in vitro. They proliferate after spinal cord injury and produce neurons in lower vertebrates but predominantly astrocytes in mammals. The mechanisms underlying this glial-biased differentiation remain ill-defined. We addressed this issue by generating a molecular resource through RNA profiling of ependymal cells before and after injury. We found that these cells activate STAT3 and ERK/MAPK signaling post injury and downregulate cilia-associated genes and FOXJ1, a central transcription factor in ciliogenesis. Conversely, they upregulate 510 genes, seven of them more than 20-fold, namely Crym, Ecm1, Ifi202b, Nupr1, Rbp1, Thbs2 and Osmr—the receptor for oncostatin, a microglia-specific cytokine which too is strongly upregulated after injury. We studied the regulation and role of Osmr using neurospheres derived from the adult spinal cord. We found that oncostatin induced strong Osmr and p-STAT3 expression in these cells which is associated with reduction of proliferation and promotion of astrocytic versus oligodendrocytic differentiation. Microglial cells are apposed to ependymal cells in vivo and co-culture experiments showed that these cells upregulate Osmr in neurosphere cultures. Collectively, these results support the notion that microglial cells and Osmr/Oncostatin pathway may regulate the astrocytic fate of ependymal cells in spinal cord injury.
Full article
►▼
Show Figures
Open AccessReview
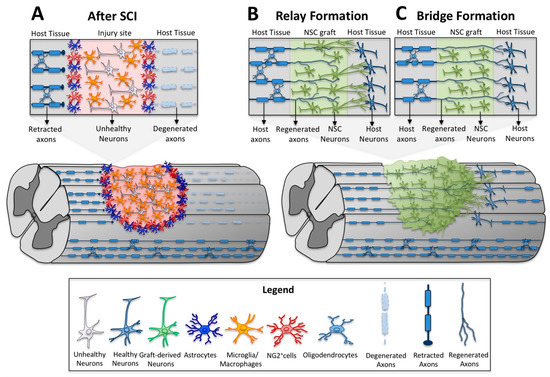
Neural Stem Cells: Promoting Axonal Regeneration and Spinal Cord Connectivity
by
Camila Marques de Freria, Erna Van Niekerk, Armin Blesch and Paul Lu
Cited by 26 | Viewed by 4391
Abstract
Spinal cord injury (SCI) leads to irreversible functional impairment caused by neuronal loss and the disruption of neuronal connections across the injury site. While several experimental strategies have been used to minimize tissue damage and to enhance axonal growth and regeneration, the corticospinal
[...] Read more.
Spinal cord injury (SCI) leads to irreversible functional impairment caused by neuronal loss and the disruption of neuronal connections across the injury site. While several experimental strategies have been used to minimize tissue damage and to enhance axonal growth and regeneration, the corticospinal projection, which is the most important voluntary motor system in humans, remains largely refractory to regenerative therapeutic interventions. To date, one of the most promising pre-clinical therapeutic strategies has been neural stem cell (NSC) therapy for SCI. Over the last decade we have found that host axons regenerate into spinal NSC grafts placed into sites of SCI. These regenerating axons form synapses with the graft, and the graft in turn extends very large numbers of new axons from the injury site over long distances into the distal spinal cord. Here we discuss the pathophysiology of SCI that makes the spinal cord refractory to spontaneous regeneration, the most recent findings of neural stem cell therapy for SCI, how it has impacted motor systems including the corticospinal tract and the implications for sensory feedback.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Spinal Cord Injury Management through the Combination of Stem Cells and Implantable 3D Bioprinted Platforms
by
Atefeh Zarepour, Sara Hooshmand, Aylin Gökmen, Ali Zarrabi and Ebrahim Mostafavi
Cited by 13 | Viewed by 4971
Abstract
Spinal cord injury (SCI) has a major impact on affected patients due to its pathological consequences and absence of capacity for self-repair. Currently available therapies are unable to restore lost neural functions. Thus, there is a pressing need to develop novel treatments that
[...] Read more.
Spinal cord injury (SCI) has a major impact on affected patients due to its pathological consequences and absence of capacity for self-repair. Currently available therapies are unable to restore lost neural functions. Thus, there is a pressing need to develop novel treatments that will promote functional repair after SCI. Several experimental approaches have been explored to tackle SCI, including the combination of stem cells and 3D bioprinting. Implanted multipotent stem cells with self-renewing capacity and the ability to differentiate to a diversity of cell types are promising candidates for replacing dead cells in injured sites and restoring disrupted neural circuits. However, implanted stem cells need protection from the inflammatory agents in the injured area and support to guide them to appropriate differentiation. Not only are 3D bioprinted scaffolds able to protect stem cells, but they can also promote their differentiation and functional integration at the site of injury. In this review, we showcase some recent advances in the use of stem cells for the treatment of SCI, different types of 3D bioprinting methods, and the combined application of stem cells and 3D bioprinting technique for effective repair of SCI.
Full article
►▼
Show Figures
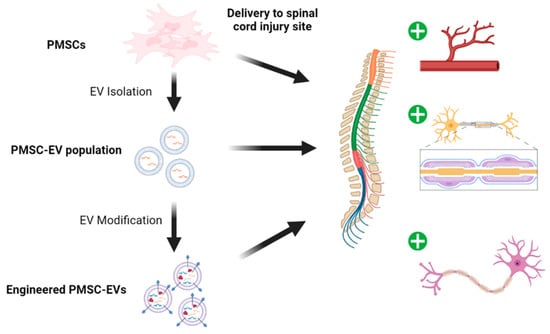
Open AccessReview
The Unique Properties of Placental Mesenchymal Stromal Cells: A Novel Source of Therapy for Congenital and Acquired Spinal Cord Injury
by
Edwin S Kulubya, Kaitlin Clark, Dake Hao, Sabrina Lazar, Arash Ghaffari-Rafi, Tejas Karnati, Julius Okudu Ebinu, Marike Zwienenberg, Diana L Farmer and Aijun Wang
Cited by 9 | Viewed by 4736
Abstract
Spinal cord injury (SCI) is a devasting condition with no reliable treatment. Spina bifida is the most common cause of congenital SCI. Cell-based therapies using mesenchymal stem/stromal cells (MSCS) have been largely utilized in SCI. Several clinical trials for acquired SCI use adult
[...] Read more.
Spinal cord injury (SCI) is a devasting condition with no reliable treatment. Spina bifida is the most common cause of congenital SCI. Cell-based therapies using mesenchymal stem/stromal cells (MSCS) have been largely utilized in SCI. Several clinical trials for acquired SCI use adult tissue-derived MSC sources, including bone-marrow, adipose, and umbilical cord tissues. The first stem/stromal cell clinical trial for spina bifida is currently underway (NCT04652908). The trial uses early gestational placental-derived mesenchymal stem/stromal cells (PMSCs) during the fetal repair of myelomeningocele. PMSCs have been shown to exhibit unique neuroprotective, angiogenic, and antioxidant properties, all which are promising applications for SCI. This review will summarize the unique properties and current applications of PMSCs and discuss their therapeutic role for acquired SCI.
Full article
►▼
Show Figures
Open AccessReview
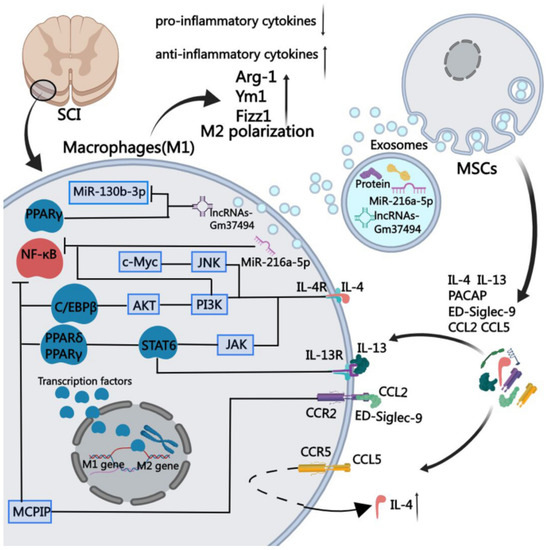
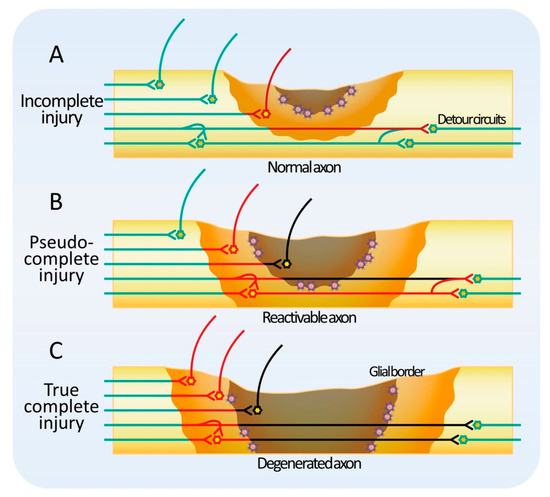
Mechanisms of Stem Cell Therapy in Spinal Cord Injuries
by
Munehisa Shinozaki, Narihito Nagoshi, Masaya Nakamura and Hideyuki Okano
Cited by 25 | Viewed by 4952
Abstract
Every year, 0.93 million people worldwide suffer from spinal cord injury (SCI) with irretrievable sequelae. Rehabilitation, currently the only available treatment, does not restore damaged tissues; therefore, the functional recovery of patients remains limited. The pathophysiology of spinal cord injuries is heterogeneous, implying
[...] Read more.
Every year, 0.93 million people worldwide suffer from spinal cord injury (SCI) with irretrievable sequelae. Rehabilitation, currently the only available treatment, does not restore damaged tissues; therefore, the functional recovery of patients remains limited. The pathophysiology of spinal cord injuries is heterogeneous, implying that potential therapeutic targets differ depending on the time of injury onset, the degree of injury, or the spinal level of injury. In recent years, despite a significant number of clinical trials based on various types of stem cells, these aspects of injury have not been effectively considered, resulting in difficult outcomes of trials. In a specialty such as cancerology, precision medicine based on a patient’s characteristics has brought indisputable therapeutic advances. The objective of the present review is to promote the development of precision medicine in the field of SCI. Here, we first describe the multifaceted pathophysiology of SCI, with the temporal changes after injury, the characteristics of the chronic phase, and the subtypes of complete injury. We then detail the appropriate targets and related mechanisms of the different types of stem cell therapy for each pathological condition. Finally, we highlight the great potential of stem cell therapy in cervical SCI.
Full article
►▼
Show Figures
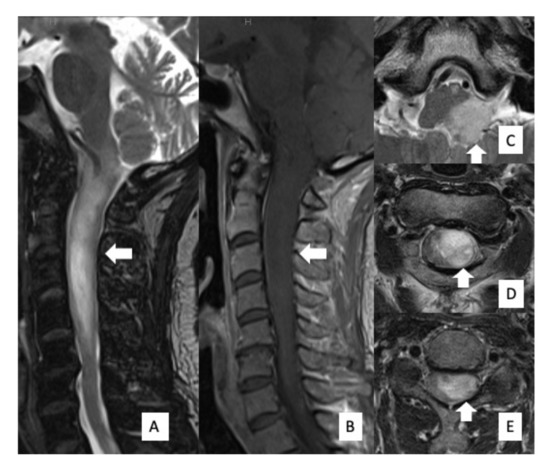
Open AccessArticle
Driver Genetic Mutations in Spinal Cord Gliomas Direct the Degree of Functional Impairment in Tumor-Associated Spinal Cord Injury
by
Yoshitaka Nagashima, Yusuke Nishimura, Fumiharu Ohka, Kaoru Eguchi, Kosuke Aoki, Hiroshi Ito, Tomoya Nishii, Takahiro Oyama, Masahito Hara, Yotaro Kitano, Hirano Masaki, Toshihiko Wakabayashi and Atsushi Natsume
Cited by 6 | Viewed by 2115
Abstract
Genetic analysis in glioma has been developed recently. Spinal cord glioma is less common than intracranial glioma. Thus, the clinical significance of genetic mutations in spinal cord gliomas remains unclear. Furthermore, because the spinal cord is an important communication channel between the brain
[...] Read more.
Genetic analysis in glioma has been developed recently. Spinal cord glioma is less common than intracranial glioma. Thus, the clinical significance of genetic mutations in spinal cord gliomas remains unclear. Furthermore, because the spinal cord is an important communication channel between the brain and the rest of the body, increased attention should be paid to its functional prognosis. In this study, we investigated the functional prognosis and driver genetic mutations in eight patients with spinal cord gliomas (World Health Organization grade I, three cases; grade II, two cases; grade III/IV, three cases).
IDH mutations were detected in all grade II cases and
H3F3A mutations were detected in all grade III/IV cases. The functional status of grade I and II gliomas remained unchanged or improved 1 year after surgery, whereas grade III/IV gliomas remained unchanged or deteriorated. Spinal glioma progenitor cells with
H3F3A mutations were associated with accelerated tumor-associated spinal cord injury, which led to functional impairment. Conversely, the presence of
IDH mutations, which are rarely reported in spinal gliomas, indicated a relatively favorable functional prognosis.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
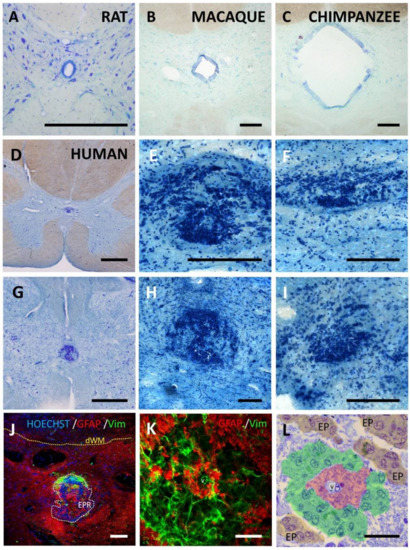
The Structure of the Spinal Cord Ependymal Region in Adult Humans Is a Distinctive Trait among Mammals
by
Alejandro Torrillas de la Cal, Beatriz Paniagua-Torija, Angel Arevalo-Martin, Christopher Guy Faulkes, Antonio Jesús Jiménez, Isidre Ferrer, Eduardo Molina-Holgado and Daniel Garcia-Ovejero
Cited by 7 | Viewed by 3800
Abstract
In species that regenerate the injured spinal cord, the ependymal region is a source of new cells and a prominent coordinator of regeneration. In mammals, cells at the ependymal region proliferate in normal conditions and react after injury, but in humans, the central
[...] Read more.
In species that regenerate the injured spinal cord, the ependymal region is a source of new cells and a prominent coordinator of regeneration. In mammals, cells at the ependymal region proliferate in normal conditions and react after injury, but in humans, the central canal is lost in the majority of individuals from early childhood. It is replaced by a structure that does not proliferate after damage and is formed by large accumulations of ependymal cells, strong astrogliosis and perivascular pseudo-rosettes. We inform here of two additional mammals that lose the central canal during their lifetime: the Naked Mole-Rat (NMR,
Heterocephalus glaber) and the mutant hyh (
hydrocephalus with hop gait) mice. The morphological study of their spinal cords shows that the tissue substituting the central canal is not similar to that found in humans. In both NMR and hyh mice, the central canal is replaced by tissue reminiscent of normal lamina X and may include small groups of ependymal cells in the midline, partially resembling specific domains of the former canal. However, no features of the adult human ependymal remnant are found, suggesting that this structure is a specific human trait. In order to shed some more light on the mechanism of human central canal closure, we provide new data suggesting that canal patency is lost by delamination of the ependymal epithelium, in a process that includes apical polarity loss and the expression of signaling mediators involved in epithelial to mesenchymal transitions.
Full article
►▼
Show Figures