Advances in Neurodegenerative Disease
Share This Topical Collection
Editors
 Prof. Dr. Pyotr A. Slominsky
Prof. Dr. Pyotr A. Slominsky
 Prof. Dr. Pyotr A. Slominsky
Prof. Dr. Pyotr A. Slominsky
E-Mail
Website
Collection Editor
Institute of Molecular Genetics of National Research Centre “Kurchatov Institute”, 2 Kurchatova Sq., 123182 Moscow, Russia
Interests: neurology; inherited disorders; genetics; DNA markers; mutations
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
I would like to devote this issue of the journal Cells to a variety of issues related to the study of human neurodegenerative diseases—from the study of the genetic and molecular mechanisms of their pathogenesis to new approaches to therapy. At the same time, attention should be given to the most common neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease. However, this does not mean that there will be no place for other diseases in this issue—for example, amyotrophic lateral sclerosis or spinocerebellar ataxia. It is only important that the articles of the Topical Collection be of interest to a wide range of scientists and clinicians, consider important aspects of the biology of neurodegenerative diseases, and make a significant contribution to their study. This Topical Collection of Cells should improve our understanding of neurodegenerative disease pathogenesis and possible approaches to early diagnostics and treatment.
Prof. Pyotr A. Slominsky
Dr. Maria Shadrina
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Neurodegenerative disease
- Genetics
- Molecular mechanisms
- Early diagnostics
- Novel therapeutics
Published Papers (23 papers)
Open AccessReview
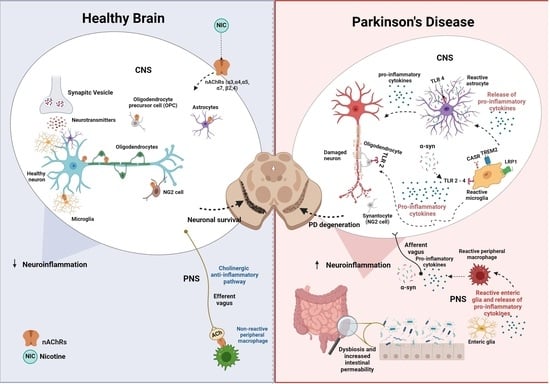
Nicotinic Acetylcholine Receptors in Glial Cells as Molecular Target for Parkinson’s Disease
by
Érica Novaes Soares, Ana Carla dos Santos Costa, Gabriel de Jesus Ferrolho, Rodrigo Portes Ureshino, Bruk Getachew, Silvia Lima Costa, Victor Diogenes Amaral da Silva and Yousef Tizabi
Viewed by 790
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by resting tremor, bradykinesia, rigidity, and postural instability that also includes non-motor symptoms such as mood dysregulation. Dopamine (DA) is the primary neurotransmitter involved in this disease, but cholinergic imbalance has also been implicated.
[...] Read more.
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by resting tremor, bradykinesia, rigidity, and postural instability that also includes non-motor symptoms such as mood dysregulation. Dopamine (DA) is the primary neurotransmitter involved in this disease, but cholinergic imbalance has also been implicated. Current intervention in PD is focused on replenishing central DA, which provides remarkable temporary symptomatic relief but does not address neuronal loss and the progression of the disease. It has been well established that neuronal nicotinic cholinergic receptors (nAChRs) can regulate DA release and that nicotine itself may have neuroprotective effects. Recent studies identified nAChRs in nonneuronal cell types, including glial cells, where they may regulate inflammatory responses. Given the crucial role of neuroinflammation in dopaminergic degeneration and the involvement of microglia and astrocytes in this response, glial nAChRs may provide a novel therapeutic target in the prevention and/or treatment of PD. In this review, following a brief discussion of PD, we focus on the role of glial cells and, specifically, their nAChRs in PD pathology and/or treatment.
Full article
►▼
Show Figures
Open AccessArticle
Progranulin Protects against Hyperglycemia-Induced Neuronal Dysfunction through GSK3β Signaling
by
Cass Dedert, Lyuba Salih and Fenglian Xu
Viewed by 1106
Abstract
Type II diabetes affects over 530 million individuals worldwide and contributes to a host of neurological pathologies. Uncontrolled high blood glucose (hyperglycemia) is a major factor in diabetic pathology, and glucose regulation is a common goal for maintenance in patients. We have found
[...] Read more.
Type II diabetes affects over 530 million individuals worldwide and contributes to a host of neurological pathologies. Uncontrolled high blood glucose (hyperglycemia) is a major factor in diabetic pathology, and glucose regulation is a common goal for maintenance in patients. We have found that the neuronal growth factor progranulin protects against hyperglycemic stress in neurons, and although its mechanism of action is uncertain, our findings identified Glycogen Synthase Kinase 3β (GSK3β) as being potentially involved in its effects. In this study, we treated mouse primary cortical neurons exposed to high-glucose conditions with progranulin and a selective pharmacological inhibitor of GSK3β before assessing neuronal health and function. Whole-cell and mitochondrial viability were both improved by progranulin under high-glucose stress in a GSK3β—dependent manner. This extended to autophagy flux, indicated by the expressions of autophagosome marker Light Chain 3B (LC3B) and lysosome marker Lysosome-Associated Membrane Protein 2A (LAMP2A), which were affected by progranulin and showed heterogeneous changes from GSK3β inhibition. Lastly, GSK3β inhibition attenuated downstream calcium signaling and neuronal firing effects due to acute progranulin treatment. These data indicate that GSK3β plays an important role in progranulin’s neuroprotective effects under hyperglycemic stress and serves as a jumping-off point to explore progranulin’s protective capabilities in other neurodegenerative models.
Full article
►▼
Show Figures
Open AccessArticle
The Pesticide Chlordecone Promotes Parkinsonism-like Neurodegeneration with Tau Lesions in Midbrain Cultures and C. elegans Worms
by
Valeria Parrales-Macias, Patrick P. Michel, Aurore Tourville, Rita Raisman-Vozari, Stéphane Haïk, Stéphane Hunot, Nicolas Bizat and Annie Lannuzel
Cited by 1 | Viewed by 2470
Abstract
Chlordecone (CLD) is an organochlorine pesticide (OCP) that is currently banned but still contaminates ecosystems in the French Caribbean. Because OCPs are known to increase the risk of Parkinson’s disease (PD), we tested whether chronic low-level intoxication with CLD could reproduce certain key
[...] Read more.
Chlordecone (CLD) is an organochlorine pesticide (OCP) that is currently banned but still contaminates ecosystems in the French Caribbean. Because OCPs are known to increase the risk of Parkinson’s disease (PD), we tested whether chronic low-level intoxication with CLD could reproduce certain key characteristics of Parkinsonism-like neurodegeneration. For that, we used culture systems of mouse midbrain dopamine (DA) neurons and glial cells, together with the nematode
C. elegans as an in vivo model organism. We established that CLD kills cultured DA neurons in a concentration- and time-dependent manner while exerting no direct proinflammatory effects on glial cells. DA cell loss was not impacted by the degree of maturation of the culture. The use of fluorogenic probes revealed that CLD neurotoxicity was the consequence of oxidative stress-mediated insults and mitochondrial disturbances. In
C. elegans worms, CLD exposure caused a progressive loss of DA neurons associated with locomotor deficits secondary to alterations in food perception. L-DOPA, a molecule used for PD treatment, corrected these deficits. Cholinergic and serotoninergic neuronal cells were also affected by CLD in
C. elegans, although to a lesser extent than DA neurons. Noticeably, CLD also promoted the phosphorylation of the aggregation-prone protein tau (but not of α-synuclein) both in midbrain cell cultures and in a transgenic
C. elegans strain expressing a human form of tau in neurons. In summary, our data suggest that CLD is more likely to promote atypical forms of Parkinsonism characterized by tau pathology than classical synucleinopathy-associated PD.
Full article
►▼
Show Figures
Open AccessArticle
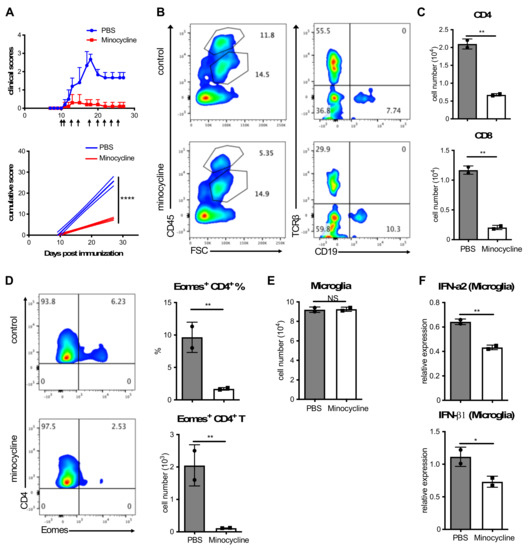
Pathogenic Microglia Orchestrate Neurotoxic Properties of Eomes-Expressing Helper T Cells
by
Chenyang Zhang, Ben Raveney, Fumio Takahashi, Tzu-wen Yeh, Hirohiko Hohjoh, Takashi Yamamura and Shinji Oki
Cited by 3 | Viewed by 2450
Abstract
In addition to disease-associated microglia (DAM), microglia with MHC-II and/or IFN-I signatures may form additional pathogenic subsets that are relevant to neurodegeneration. However, the significance of such MHC-II and IFN-I signatures remains elusive. We demonstrate here that these microglial subsets play intrinsic roles
[...] Read more.
In addition to disease-associated microglia (DAM), microglia with MHC-II and/or IFN-I signatures may form additional pathogenic subsets that are relevant to neurodegeneration. However, the significance of such MHC-II and IFN-I signatures remains elusive. We demonstrate here that these microglial subsets play intrinsic roles in orchestrating neurotoxic properties of neurotoxic Eomes
+ Th cells under the neurodegeneration-associated phase of experimental autoimmune encephalomyelitis (EAE) that corresponds to progressive multiple sclerosis (MS). Microglia acquire IFN-signature after sensing ectopically expressed long interspersed nuclear element-1 (L1) gene. Furthermore, ORF1, an L1-encoded protein aberrantly expressed in the diseased central nervous system (CNS), stimulated Eomes
+ Th cells after Trem2-dependent ingestion and presentation in MHC-II context by microglia. Interestingly, administration of an L1 inhibitor significantly ameliorated neurodegenerative symptoms of EAE concomitant with reduced accumulation of Eomes
+ Th cells in the CNS. Collectively, our data highlight a critical contribution of new microglia subsets as a neuroinflammatory hub in immune-mediated neurodegeneration.
Full article
►▼
Show Figures
Open AccessArticle
Sex-Specific Microglial Responses to Glucocerebrosidase Inhibition: Relevance to GBA1-Linked Parkinson’s Disease
by
Electra Brunialti, Alessandro Villa, Marco Toffoli, Sara Lucas Del Pozo, Nicoletta Rizzi, Clara Meda, Adriana Maggi, Anthony H. V. Schapira and Paolo Ciana
Cited by 2 | Viewed by 2017
Abstract
Microglia are heterogenous cells characterized by distinct populations each contributing to specific biological processes in the nervous system, including neuroprotection. To elucidate the impact of sex-specific microglia heterogenicity to the susceptibility of neuronal stress, we video-recorded with time-lapse microscopy the changes in shape
[...] Read more.
Microglia are heterogenous cells characterized by distinct populations each contributing to specific biological processes in the nervous system, including neuroprotection. To elucidate the impact of sex-specific microglia heterogenicity to the susceptibility of neuronal stress, we video-recorded with time-lapse microscopy the changes in shape and motility occurring in primary cells derived from mice of both sexes in response to pro-inflammatory or neurotoxic stimulations. With this morpho-functional analysis, we documented distinct microglia subpopulations eliciting sex-specific responses to stimulation: male microglia tended to have a more pro-inflammatory phenotype, while female microglia showed increased sensitivity to conduritol-B-epoxide (CBE), a small molecule inhibitor of glucocerebrosidase, the enzyme encoded by the GBA1 gene, mutations of which are the major risk factor for Parkinson’s Disease (PD). Interestingly, glucocerebrosidase inhibition particularly impaired the ability of female microglia to enhance the Nrf2-dependent detoxification pathway in neurons, attenuating the sex differences observed in this neuroprotective function. This finding is consistent with the clinical impact of GBA1 mutations, in which the 1.5–2-fold reduced risk of developing idiopathic PD observed in female individuals is lost in the GBA1 carrier population, thus suggesting a sex-specific role for microglia in the etiopathogenesis of PD-GBA1.
Full article
►▼
Show Figures
Open AccessArticle
A Single Chain Fragment Variant Binding Misfolded Alpha-Synuclein Exhibits Neuroprotective and Antigen-Specific Anti-Inflammatory Properties
by
Michael Fassler, Clara Benaim and Jacob George
Cited by 4 | Viewed by 1627
Abstract
Introduction. Alpha synuclein (αSyn) misfolding plays a requisite role in the pathogenesis of synucleinopathies. Direct toxicity to neurons, triggering neuroinflammation as well as the spreading and seeding of αSyn pathology are essential pathogenetic underlying mechanisms. Immunotherapy in experimental Parkinson’s disease (PD) has been
[...] Read more.
Introduction. Alpha synuclein (αSyn) misfolding plays a requisite role in the pathogenesis of synucleinopathies. Direct toxicity to neurons, triggering neuroinflammation as well as the spreading and seeding of αSyn pathology are essential pathogenetic underlying mechanisms. Immunotherapy in experimental Parkinson’s disease (PD) has been shown to be consistently effective in preclinical models, yet the initial clinical trials with monoclonal antibodies (mAbs) yielded marginal results if any. Aiming to overcome some of the limitation of this approach, we aimed to select an αSyn binding scFv antibody format and test it in multiple experimental PD in vivo models. Methods. We cloned the lead αSyn scFv based on preselection of human phage display libraries of human Fab. The selected of scFv targeting both oligomers and pre-formed fibrils (PFF) of αSyn were tested for their ability to protect neurons from triggered toxicity, influence their uptake to microglia, and accelerate misfolded αSyn degradation. The lead scFv- sMB08, was also tested for its ability to impact αSyn aggregation as well as spreading and seeding. Results. sMB08 was shown to protect neurons from misfolded αSyn mediated toxicity, promote its intracellular degradation, and to reduce its uptake by microglia. sMB08 exhibited anti-inflammatory properties, including its ability to attenuate adaptive αSyn autoimmunity and ameliorate proinflammatory cytokine expression in brains of mice stereotactically injected with PFF. Employing three experimental models of PD, intranasal treatment with sMB08 attenuated motoric dysfunction and achieved acceptable brain levels by pharmacokinetic analysis, leading to significant preservation of dopaminergic n neurons. Conclusion: sMB08, a scFv targeting both αSyn oligomers and PFF, due to its small size facilitating paraneural brain penetration and avoidance of nonspecific inflammation, appears as an attractive approach to test in patients with PD by addressing the major mechanisms that mediate misfolded αSyn driven pathology.
Full article
►▼
Show Figures
Open AccessArticle
Neuroprotective Effects of Neuropeptide Y on Human Neuroblastoma SH-SY5Y Cells in Glutamate Excitotoxicity and ER Stress Conditions
by
Viswanthram Palanivel, Vivek Gupta, Seyed Shahab Oddin Mirshahvaladi, Samridhi Sharma, Veer Gupta, Nitin Chitranshi, Mehdi Mirzaei, Stuart L Graham and Devaraj Basavarajappa
Cited by 4 | Viewed by 2387
Abstract
Neuropeptide Y (NPY), a sympathetic neurotransmitter, is involved in various physiological functions, and its dysregulation is implicated in several neurodegenerative diseases. Glutamate excitotoxicity, endoplasmic reticulum (ER) stress, and oxidative stress are the common mechanisms associated with numerous neurodegenerative illnesses. The present study aimed
[...] Read more.
Neuropeptide Y (NPY), a sympathetic neurotransmitter, is involved in various physiological functions, and its dysregulation is implicated in several neurodegenerative diseases. Glutamate excitotoxicity, endoplasmic reticulum (ER) stress, and oxidative stress are the common mechanisms associated with numerous neurodegenerative illnesses. The present study aimed to elucidate the protective effects of NPY against glutamate toxicity and tunicamycin-induced ER stress in the human neuroblastoma SH-SY5Y cell line. We exposed the SH-SY5Y cells to glutamate and tunicamycin for two different time points and analyzed the protective effects of NPY at different concentrations. The protective effects of NPY treatments were assessed by cell viability assay, and the signalling pathway changes were evaluated by biochemical techniques such as Western blotting and immunofluorescence assays. Our results showed that treatment of SH-SY5Y cells with NPY significantly increased the viability of the cells in both glutamate toxicity and ER stress conditions. NPY treatments significantly attenuated the glutamate-induced pro-apoptotic activation of ERK1/2 and JNK/BAD pathways. The protective effects of NPY were further evident against tunicamycin-induced ER stress. NPY treatments significantly suppressed the ER stress activation by downregulating BiP, phospho-eIF2α, and CHOP expression. In addition, NPY alleviated the Akt/FoxO3a pathway in acute oxidative conditions caused by glutamate and tunicamycin in SH-SY5Y cells. Our results demonstrated that NPY is neuroprotective against glutamate-induced cell toxicity and tunicamycin-induced ER stress through anti-apoptotic actions.
Full article
►▼
Show Figures
Open AccessArticle
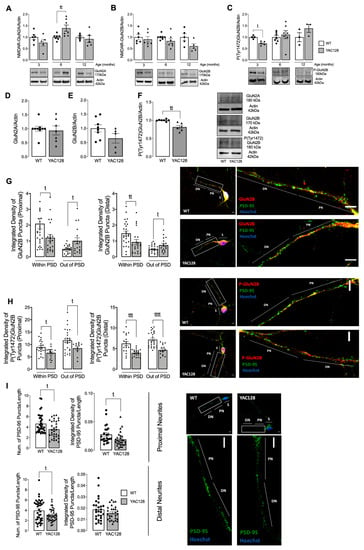
Restored Fyn Levels in Huntington’s Disease Contributes to Enhanced Synaptic GluN2B-Composed NMDA Receptors and CREB Activity
by
Lígia Fão, Patrícia Coelho, Ricardo J. Rodrigues and A. Cristina Rego
Cited by 3 | Viewed by 1533
Abstract
N-methyl-D-aspartate receptors (NMDARs) are important postsynaptic receptors that contribute to normal synaptic function and cell survival; however, when overactivated, as in Huntington’s disease (HD), NMDARs cause excitotoxicity. HD-affected striatal neurons show altered NMDAR currents and augmented ratio of surface to internal GluN2B-containing
[...] Read more.
N-methyl-D-aspartate receptors (NMDARs) are important postsynaptic receptors that contribute to normal synaptic function and cell survival; however, when overactivated, as in Huntington’s disease (HD), NMDARs cause excitotoxicity. HD-affected striatal neurons show altered NMDAR currents and augmented ratio of surface to internal GluN2B-containing NMDARs, with augmented accumulation at extrasynaptic sites. Fyn protein is a member of the Src kinase family (SKF) with an important role in NMDARs phosphorylation and synaptic localization and function; recently, we demonstrated that Fyn is reduced in several HD models. Thus, in this study, we aimed to explore the impact of HD-mediated altered Fyn levels at post-synaptic density (PSD), and their role in distorted NMDARs function and localization, and intracellular neuroprotective pathways in YAC128 mouse primary striatal neurons. We show that reduced synaptic Fyn levels and activity in HD mouse striatal neurons is related to decreased phosphorylation of synaptic GluN2B-composed NMDARs; this occurs concomitantly with augmented extrasynaptic NMDARs activity and currents and reduced cAMP response element-binding protein (CREB) activation, along with induction of cell death pathways. Importantly, expression of a constitutive active form of SKF reestablishes NMDARs localization, phosphorylation, and function at PSD in YAC128 mouse neurons. Enhanced SKF levels and activity also promotes CREB activation and reduces caspase-3 activation in YAC128 mouse striatal neurons. This work supports, for the first time, a relevant role for Fyn protein in PSD modulation, controlling NMDARs synaptic function in HD, and favoring neuroprotective pathways and cell survival. In this respect, Fyn Tyr kinase constitutes an important potential HD therapeutic target directly acting at PSD.
Full article
►▼
Show Figures
Open AccessArticle
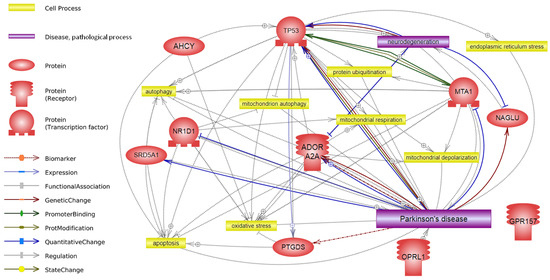
Transcriptome Profiling Reveals Differential Expression of Circadian Behavior Genes in Peripheral Blood of Monozygotic Twins Discordant for Parkinson’s Disease
by
Ekaterina I. Semenova, Ivan N. Vlasov, Suzanna A. Partevian, Anna V. Rosinskaya, Ivan N. Rybolovlev, Petr A. Slominsky, Maria I. Shadrina and Anelya Kh. Alieva
Cited by 1 | Viewed by 1849
Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Investigating individuals with the most identical genetic background is optimal for minimizing the genetic contribution to gene expression. These individuals include monozygotic twins discordant for PD. Monozygotic twins have the same genetic
[...] Read more.
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Investigating individuals with the most identical genetic background is optimal for minimizing the genetic contribution to gene expression. These individuals include monozygotic twins discordant for PD. Monozygotic twins have the same genetic background, age, sex, and often similar environmental conditions. The aim of this study was to carry out a transcriptome analysis of the peripheral blood of three pairs of monozygotic twins discordant for PD. We identified the metabolic process “circadian behavior” as a priority process for further study. Different expression of genes included in the term “circadian behavior” confirms that this process is involved in PD pathogenesis. We found increased expression of three genes associated with circadian behavior, i.e.,
PTGDS,
ADORA2A, and
MTA1, in twins with PD. These genes can be considered as potential candidate genes for this disease.
Full article
►▼
Show Figures
Open AccessArticle
Suppression of Linear Ubiquitination Ameliorates Cytoplasmic Aggregation of Truncated TDP-43
by
Qiang Zhang, Seigo Terawaki, Daisuke Oikawa, Yoshinori Okina, Yoshinosuke Usuki, Hidefumi Ito and Fuminori Tokunaga
Cited by 4 | Viewed by 2495
Abstract
TAR DNA-binding protein 43 (TDP-43) is a predominant component of inclusions in the brains and spines of patients with amyotrophic lateral sclerosis (ALS). The progressive accumulation of inclusions leads to proteinopathy in neurons. We have previously shown that Met1(M1)-linked linear ubiquitin, which is
[...] Read more.
TAR DNA-binding protein 43 (TDP-43) is a predominant component of inclusions in the brains and spines of patients with amyotrophic lateral sclerosis (ALS). The progressive accumulation of inclusions leads to proteinopathy in neurons. We have previously shown that Met1(M1)-linked linear ubiquitin, which is specifically generated by the linear ubiquitin chain assembly complex (LUBAC), is colocalized with TDP-43 inclusions in neurons from
optineurin-associated familial and sporadic ALS patients, and affects NF-κB activation and apoptosis. To examine the effects of LUBAC-mediated linear ubiquitination on TDP-43 proteinopathies, we performed cell biological analyses using full-length and truncated forms of the ALS-associated Ala315→Thr (A315T) mutant of TDP-43 in Neuro2a cells. The truncated A315T mutants of TDP-43, which lack a nuclear localization signal, efficiently generated cytoplasmic aggregates that were colocalized with multiple ubiquitin chains such as M1-, Lys(K)48-, and K63-chains. Genetic ablation of
HOIP or treatment with a LUBAC inhibitor, HOIPIN-8, suppressed the cytoplasmic aggregation of A315T mutants of TDP-43. Moreover, the enhanced TNF-α-mediated NF-κB activity by truncated TDP-43 mutants was eliminated in the presence of HOIPIN-8. These results suggest that multiple ubiquitinations of TDP-43 including M1-ubiquitin affect protein aggregation and inflammatory responses in vitro, and therefore, LUBAC inhibition ameliorates TDP-43 proteinopathy.
Full article
►▼
Show Figures
Open AccessArticle
Cannabidiol Inhibits Tau Aggregation In Vitro
by
Soha Alali, Gholamhossein Riazi, Mohammad Reza Ashrafi-Kooshk, Sogol Meknatkhah, Shahin Ahmadian, Mohammad Hooshyari Ardakani and Baharak Hosseinkhani
Cited by 12 | Viewed by 4951
Abstract
A hallmark of Alzheimer’s disease (AD) is the accumulation of tau protein in the brain. Compelling evidence indicates that the presence of tau aggregates causes irreversible neuronal destruction, eventually leading to synaptic loss. So far, the inhibition of tau aggregation has been recognized
[...] Read more.
A hallmark of Alzheimer’s disease (AD) is the accumulation of tau protein in the brain. Compelling evidence indicates that the presence of tau aggregates causes irreversible neuronal destruction, eventually leading to synaptic loss. So far, the inhibition of tau aggregation has been recognized as one of the most effective therapeutic strategies. Cannabidiol (CBD), a major component found in
Cannabis sativa L., has antioxidant activities as well as numerous neuroprotective features. Therefore, we hypothesize that CBD may serve as a potent substance to hamper tau aggregation in AD. In this study, we aim to investigate the CBD effect on the aggregation of recombinant human tau protein 1N/4R isoform using biochemical methods in vitro and in silico. Using Thioflavin T (ThT) assay, circular dichroism (CD), and atomic force microscopy (AFM), we demonstrated that CBD can suppress tau fibrils formation. Moreover, by quenching assay, docking, and job’s plot, we further demonstrated that one molecule of CBD interacts with one molecule of tau protein through a spontaneous binding. Experiments performed by quenching assay, docking, and Thioflavin T assay further established that the main forces are hydrogen Van der Waals and some non-negligible hydrophobic forces, affecting the lag phase of tau protein kinetics. Taken together, this study provides new insights about a natural substance, CBD, for tau therapy which may offer new hope for the treatment of AD.
Full article
►▼
Show Figures
Open AccessReview
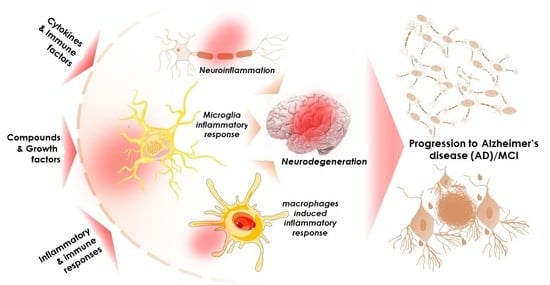
The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer’s Disease
by
Gilbert Ogunmokun, Saikat Dewanjee, Pratik Chakraborty, Chandrasekhar Valupadas, Anupama Chaudhary, Viswakalyan Kolli, Uttpal Anand, Jayalakshmi Vallamkondu, Parul Goel, Hari Prasad Reddy Paluru, Kiran Dip Gill, P. Hemachandra Reddy, Vincenzo De Feo and Ramesh Kandimalla
Cited by 30 | Viewed by 5512
Abstract
Alzheimer’s disease (AD) is one of the most prominent neurodegenerative diseases, which impairs cognitive function in afflicted individuals. AD results in gradual decay of neuronal function as a consequence of diverse degenerating events. Several neuroimmune players (such as cytokines and growth factors that
[...] Read more.
Alzheimer’s disease (AD) is one of the most prominent neurodegenerative diseases, which impairs cognitive function in afflicted individuals. AD results in gradual decay of neuronal function as a consequence of diverse degenerating events. Several neuroimmune players (such as cytokines and growth factors that are key players in maintaining CNS homeostasis) turn aberrant during crosstalk between the innate and adaptive immunities. This aberrance underlies neuroinflammation and drives neuronal cells toward apoptotic decline. Neuroinflammation involves microglial activation and has been shown to exacerbate AD. This review attempted to elucidate the role of cytokines, growth factors, and associated mechanisms implicated in the course of AD, especially with neuroinflammation. We also evaluated the propensities and specific mechanism(s) of cytokines and growth factors impacting neuron upon apoptotic decline and further shed light on the availability and accessibility of cytokines across the blood-brain barrier and choroid plexus in AD pathophysiology. The pathogenic and the protective roles of macrophage migration and inhibitory factors, neurotrophic factors, hematopoietic-related growth factors, TAU phosphorylation, advanced glycation end products, complement system, and glial cells in AD and neuropsychiatric pathology were also discussed. Taken together, the emerging roles of these factors in AD pathology emphasize the importance of building novel strategies for an effective therapeutic/neuropsychiatric management of AD in clinics.
Full article
►▼
Show Figures
Open AccessArticle
Housekeeping Genes for Parkinson’s Disease in Humans and Mice
by
Anelya Kh. Alieva, Elena V. Filatova, Margarita M. Rudenok, Petr A. Slominsky and Maria I. Shadrina
Cited by 5 | Viewed by 2946
Abstract
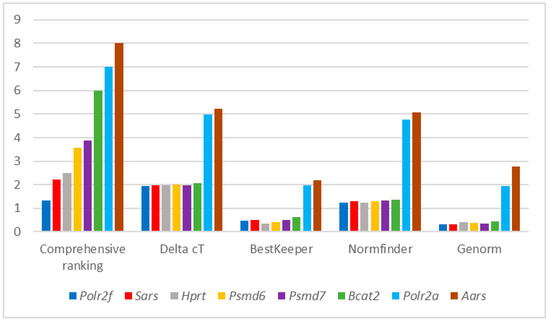
A critical aspect of real-time PCR is the presence of housekeeping genes (HKGs) as an internal control for the normalization of expression data for genes of interest. It is necessary to select correct HKGs in the investigation of various pathologies. Thereby, we analyzed
[...] Read more.
A critical aspect of real-time PCR is the presence of housekeeping genes (HKGs) as an internal control for the normalization of expression data for genes of interest. It is necessary to select correct HKGs in the investigation of various pathologies. Thereby, we analyzed the stability of expression of the HKGs in Parkinson’s disease (PD). The work was carried out in the peripheral blood of patients with PD and in the brain tissues and peripheral blood of mice with MPTP-induced PD. As a result,
Aars was the most stably expressed HKG in the mouse brain as a whole. However, different genes were more stably expressed in different parts of the brain.
Polr2f was the most stably expressed in the cortex,
Psmd6 was the most stably expressed in the cerebellum, and
Psmd7 was the most stably expressed in the striatum and substantia nigra. HKGs were different in similar tissues of the studied organisms.
Polr2f was the most stably expressed HKG in the peripheral blood of mice, whereas
PSMD6 was the most stably expressed gene in humans. Thus, there is no universal HKG both for different brain tissues of one organism and for similar tissues of different organisms. Furthermore, the identified most stably expressed HKGs can be considered as such only under conditions in PD.
Full article
►▼
Show Figures
Open AccessArticle
Identification of Novel Cathepsin B Inhibitors with Implications in Alzheimer’s Disease: Computational Refining and Biochemical Evaluation
by
Nitin Chitranshi, Ashutosh Kumar, Samran Sheriff, Veer Gupta, Angela Godinez, Danit Saks, Soumalya Sarkar, Ting Shen, Mehdi Mirzaei, Devaraj Basavarajappa, Morteza Abyadeh, Sachin K. Singh, Kamal Dua, Kam Y. J. Zhang, Stuart L. Graham and Vivek Gupta
Cited by 13 | Viewed by 3568
Abstract
Amyloid precursor protein (APP), upon proteolytic degradation, forms aggregates of amyloid β (Aβ) and plaques in the brain, which are pathological hallmarks of Alzheimer’s disease (AD). Cathepsin B is a cysteine protease enzyme that catalyzes the proteolytic degradation of APP in the brain.
[...] Read more.
Amyloid precursor protein (APP), upon proteolytic degradation, forms aggregates of amyloid β (Aβ) and plaques in the brain, which are pathological hallmarks of Alzheimer’s disease (AD). Cathepsin B is a cysteine protease enzyme that catalyzes the proteolytic degradation of APP in the brain. Thus, cathepsin B inhibition is a crucial therapeutic aspect for the discovery of new anti-Alzheimer’s drugs. In this study, we have employed mixed-feature ligand-based virtual screening (LBVS) by integrating pharmacophore mapping, docking, and molecular dynamics to detect small, potent molecules that act as cathepsin B inhibitors. The LBVS model was generated by using hydrophobic (HY), hydrogen bond acceptor (HBA), and hydrogen bond donor (HBD) features, using a dataset of 24 known cathepsin B inhibitors of both natural and synthetic origins. A validated eight-feature pharmacophore hypothesis (Hypo III) was utilized to screen the Maybridge chemical database. The docking score, MM-PBSA, and MM-GBSA methodology was applied to prioritize the lead compounds as virtual screening hits. These compounds share a common amide scaffold, and showed important interactions with Gln23, Cys29, His110, His111, Glu122, His199, and Trp221. The identified inhibitors were further evaluated for cathepsin-B-inhibitory activity. Our study suggests that pyridine, acetamide, and benzohydrazide compounds could be used as a starting point for the development of novel therapeutics.
Full article
►▼
Show Figures
Open AccessArticle
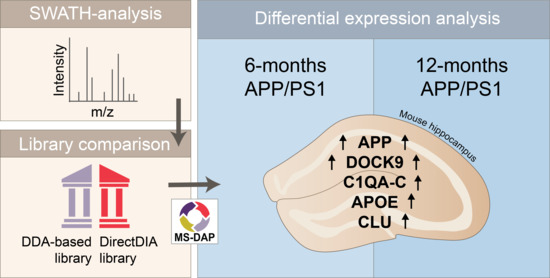
Age-Dependent Hippocampal Proteomics in the APP/PS1 Alzheimer Mouse Model: A Comparative Analysis with Classical SWATH/DIA and directDIA Approaches
by
Sophie J. F. van der Spek, Miguel A. Gonzalez-Lozano, Frank Koopmans, Suzanne S. M. Miedema, Iryna Paliukhovich, August B. Smit and Ka Wan Li
Cited by 10 | Viewed by 3041
Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the human population, for which there is currently no cure. The cause of AD is unknown; however, the toxic effects of amyloid-β (Aβ) are believed to play a role in its onset. To
[...] Read more.
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the human population, for which there is currently no cure. The cause of AD is unknown; however, the toxic effects of amyloid-β (Aβ) are believed to play a role in its onset. To investigate this, we examined changes in global protein levels in a hippocampal synaptosome fraction of the Amyloid Precursor Protein swe/Presenelin 1 dE9 (APP/PS1) mouse model of AD at 6 and 12 months of age (moa). Data independent acquisition (DIA), or Sequential Window Acquisition of all THeoretical fragment-ion (SWATH), was used for a quantitative label-free proteomics analysis. We first assessed the usefulness of a recently improved directDIA workflow as an alternative to conventional DIA data analysis using a project-specific spectral library. Subsequently, we applied directDIA to the 6- and 12-moa APP/PS1 datasets and applied the Mass Spectrometry Downstream Analysis Pipeline (MS-DAP) for differential expression analysis and candidate discovery. We observed most regulation at 12-moa, in particular of proteins involved in Aβ homeostasis and microglial-dependent processes, like synaptic pruning and the immune response, such as APOE, CLU and C1QA-C. All proteomics data are available via ProteomeXchange with identifier PXD025777.
Full article
►▼
Show Figures
Open AccessReview
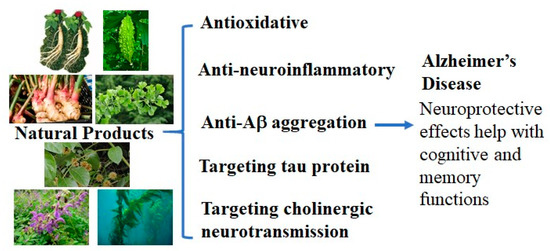
Neuroprotective Natural Products for Alzheimer’s Disease
by
Xin Chen, Joshua Drew, Wren Berney and Wei Lei
Cited by 89 | Viewed by 7482
Abstract
Alzheimer’s disease (AD) is the number one neurovegetative disease, but its treatment options are relatively few and ineffective. In efforts to discover new strategies for AD therapy, natural products have aroused interest in the research community and in the pharmaceutical industry for their
[...] Read more.
Alzheimer’s disease (AD) is the number one neurovegetative disease, but its treatment options are relatively few and ineffective. In efforts to discover new strategies for AD therapy, natural products have aroused interest in the research community and in the pharmaceutical industry for their neuroprotective activity, targeting different pathological mechanisms associated with AD. A wide variety of natural products from different origins have been evaluated preclinically and clinically for their neuroprotective mechanisms in preventing and attenuating the multifactorial pathologies of AD. This review mainly focuses on the possible neuroprotective mechanisms from natural products that may be beneficial in AD treatment and the natural product mixtures or extracts from different sources that have demonstrated neuroprotective activity in preclinical and/or clinical studies. It is believed that natural product mixtures or extracts containing multiple bioactive compounds that can work additively or synergistically to exhibit multiple neuroprotective mechanisms might be an effective approach in AD drug discovery.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
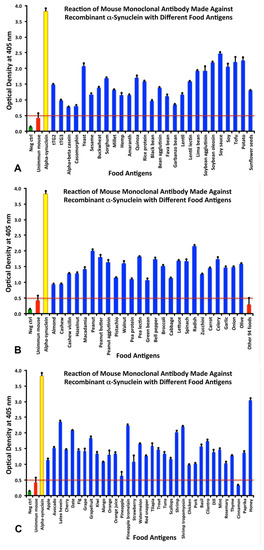
Cross-Reactivity and Sequence Homology Between Alpha-Synuclein and Food Products: A Step Further for Parkinson’s Disease Synucleinopathy
by
Aristo Vojdani, Aaron Lerner and Elroy Vojdani
Cited by 18 | Viewed by 3612
Abstract
Introduction: Parkinson’s disease is characterized by non-motor/motor dysfunction midbrain neuronal death and α-synuclein deposits. The accepted hypothesis is that unknown environmental factors induce α-synuclein accumulation in the brain via the enteric nervous system. Material and Methods: Monoclonal antibodies made against recombinant α-synuclein protein
[...] Read more.
Introduction: Parkinson’s disease is characterized by non-motor/motor dysfunction midbrain neuronal death and α-synuclein deposits. The accepted hypothesis is that unknown environmental factors induce α-synuclein accumulation in the brain via the enteric nervous system. Material and Methods: Monoclonal antibodies made against recombinant α-synuclein protein or α-synuclein epitope 118–123 were applied to the antigens of 180 frequently consumed food products. The specificity of those antibody-antigen reactions was confirmed by serial dilution and inhibition studies. The Basic Local Alignment Search Tool sequence matching program was used for sequence homologies. Results: While the antibody made against recombinant α-synuclein reacted significantly with 86/180 specific food antigens, the antibody made against α-synuclein epitope 118–123 reacted with only 32/180 tested food antigens. The food proteins with the greatest number of peptides that matched with α-synuclein were yeast, soybean, latex hevein, wheat germ agglutinin, potato, peanut, bean agglutinin, pea lectin, shrimp, bromelain, and lentil lectin.
Conclusions: The cross-reactivity and sequence homology between α-synuclein and frequently consumed foods, reinforces the autoimmune aspect of Parkinson’s disease. It is hypothesized that luminal food peptides that share cross-reactive epitopes with human α-synuclein and have molecular similarity with brain antigens are involved in the synucleinopathy. The findings deserve further confirmation by extensive research.
Full article
►▼
Show Figures
Open AccessReview
Fenamates as Potential Therapeutics for Neurodegenerative Disorders
by
Jaunetta Hill and Nasser H. Zawia
Cited by 12 | Viewed by 3976
Abstract
Neurodegenerative disorders are desperately lacking treatment options. It is imperative that drug repurposing be considered in the fight against neurodegenerative diseases. Fenamates have been studied for efficacy in treating several neurodegenerative diseases. The purpose of this review is to comprehensively present the past
[...] Read more.
Neurodegenerative disorders are desperately lacking treatment options. It is imperative that drug repurposing be considered in the fight against neurodegenerative diseases. Fenamates have been studied for efficacy in treating several neurodegenerative diseases. The purpose of this review is to comprehensively present the past and current research on fenamates in the context of neurodegenerative diseases with a special emphasis on tolfenamic acid and Alzheimer’s disease. Furthermore, this review discusses the major molecular pathways modulated by fenamates.
Full article
►▼
Show Figures
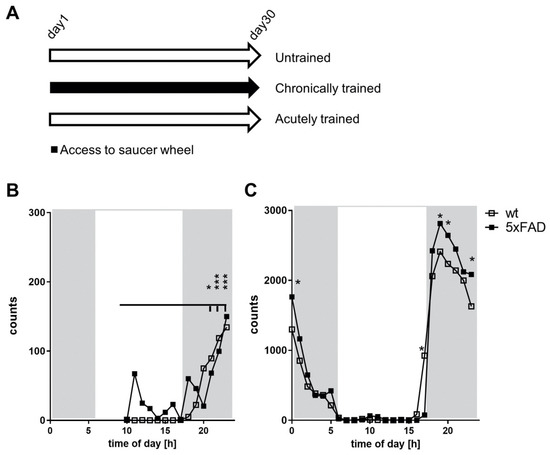
Open AccessArticle
Voluntary Wheel Running Did Not Alter Gene Expression in 5xfad Mice, but in Wild-Type Animals Exclusively after One-Day of Physical Activity
by
Anna Wierczeiko, Lena Gammel, Konstantin Radyushkin, Vu Thu Thuy Nguyen, Hristo Todorov, Susanne Gerber and Kristina Endres
Cited by 2 | Viewed by 3263
Abstract
Physical activity is considered a promising preventive intervention to reduce the risk of developing Alzheimer’s disease (AD). However, the positive effect of therapeutic administration of physical activity has not been proven conclusively yet, likely due to confounding factors such as varying activity regimens
[...] Read more.
Physical activity is considered a promising preventive intervention to reduce the risk of developing Alzheimer’s disease (AD). However, the positive effect of therapeutic administration of physical activity has not been proven conclusively yet, likely due to confounding factors such as varying activity regimens and life or disease stages. To examine the impact of different routines of physical activity in the early disease stages, we subjected young 5xFAD and wild-type mice to 1-day (acute) and 30-day (chronic) voluntary wheel running and compared them with age-matched sedentary controls. We observed a significant increase in brain lactate levels in acutely trained 5xFAD mice relative to all other experimental groups. Subsequent brain RNA-seq analysis did not reveal major differences in transcriptomic regulation between training durations in 5xFAD mice. In contrast, acute training yielded substantial gene expression changes in wild-type animals relative to their chronically trained and sedentary counterparts. The comparison of 5xFAD and wild-type mice showed the highest transcriptional differences in the chronic and sedentary groups, whereas acute training was associated with much fewer differentially expressed genes. In conclusion, our results suggest that different training durations did not affect the global transcriptome of 3-month-old 5xFAD mice, whereas acute running seemed to induce a similar transcriptional stress state in wild-type animals as already known for 5xFAD mice.
Full article
►▼
Show Figures
Open AccessReview
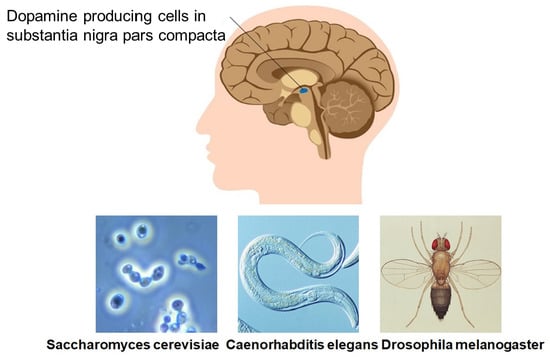
Invertebrate Models Untangle the Mechanism of Neurodegeneration in Parkinson’s Disease
by
Andrei Surguchov
Cited by 21 | Viewed by 3239
Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease, afflicting ~10 million people worldwide. Although several genes linked to PD are currently identified, PD remains primarily an idiopathic disorder. Neuronal protein α-synuclein is a major player in disease progression of both genetic
[...] Read more.
Parkinson’s disease (PD) is the second most common neurodegenerative disease, afflicting ~10 million people worldwide. Although several genes linked to PD are currently identified, PD remains primarily an idiopathic disorder. Neuronal protein α-synuclein is a major player in disease progression of both genetic and idiopathic forms of PD. However, it cannot alone explain underlying pathological processes. Recent studies demonstrate that many other risk factors can accelerate or further worsen brain dysfunction in PD patients. Several PD models, including non-mammalian eukaryotic organisms, have been developed to identify and characterize these factors. This review discusses recent findings in three PD model organisms, i.e., yeast, Drosophila, and
Caenorhabditis elegans, that opened new mechanisms and identified novel contributors to this disorder. These non-mammalian models share many conserved molecular pathways and cellular processes with humans. New players affecting PD pathogenesis include previously unknown genes/proteins, novel signaling pathways, and low molecular weight substances. These findings might respond to the urgent need to discover novel drug targets for PD treatment and new biomarkers for early diagnostics of this disease. Since the study of neurodegeneration using simple eukaryotic organisms brought a huge amount of information, we include only the most recent or the most important relevant data.
Full article
►▼
Show Figures
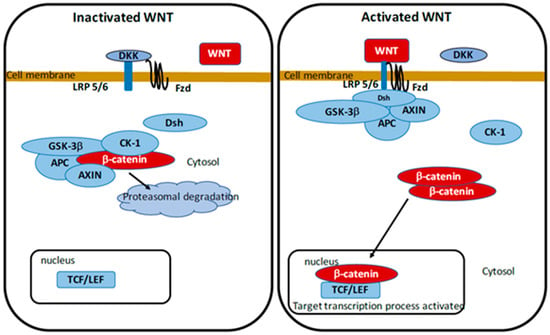
Open AccessReview
Parkinson’s Disease: Potential Actions of Lithium by Targeting the WNT/β-Catenin Pathway, Oxidative Stress, Inflammation and Glutamatergic Pathway
by
Alexandre Vallée, Jean-Noël Vallée and Yves Lecarpentier
Cited by 37 | Viewed by 5278
Abstract
Parkinson’s disease (PD) is one of the major neurodegenerative diseases (ND) which presents a progressive neurodegeneration characterized by loss of dopamine in the substantia nigra pars compacta. It is well known that oxidative stress, inflammation and glutamatergic pathway play key roles in the
[...] Read more.
Parkinson’s disease (PD) is one of the major neurodegenerative diseases (ND) which presents a progressive neurodegeneration characterized by loss of dopamine in the substantia nigra pars compacta. It is well known that oxidative stress, inflammation and glutamatergic pathway play key roles in the development of PD. However, therapies remain uncertain and research for new treatment is mandatory. This review focuses on the potential effects of lithium, as a potential therapeutic strategy, on PD and some of the presumed mechanisms by which lithium provides its benefit properties. Lithium medication downregulates GSK-3beta, the main inhibitor of the WNT/β-catenin pathway. The stimulation of the WNT/β-catenin could be associated with the control of oxidative stress, inflammation, and glutamatergic pathway. Future prospective clinical trials could focus on lithium and its different and multiple interactions in PD.
Full article
►▼
Show Figures
Open AccessArticle
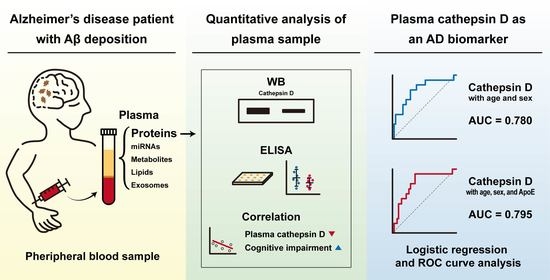
Identification of Cathepsin D as a Plasma Biomarker for Alzheimer’s Disease
by
Jae-Whan Kim, Soon-Young Jung, Youngbin Kim, Hansol Heo, Chang-Hyung Hong, Sang-Won Seo, Seong-Hye Choi, Sang-Joon Son, Seongju Lee and Jaerak Chang
Cited by 18 | Viewed by 3742
Abstract
Although Alzheimer’s disease (AD) is the most common neurodegenerative disease, there are still no drugs available to treat or prevent AD effectively. Here, we examined changes in levels of selected proteins implicated in the pathogenesis of AD using plasma samples of control subjects
[...] Read more.
Although Alzheimer’s disease (AD) is the most common neurodegenerative disease, there are still no drugs available to treat or prevent AD effectively. Here, we examined changes in levels of selected proteins implicated in the pathogenesis of AD using plasma samples of control subjects and patients with cognition impairment. To precisely categorize the disease, fifty-six participants were examined with clinical cognitive tests, amyloid positron emission tomography (PET) scan, and white matter hyperintensities scored by magnetic resonance imaging. Plasma cathepsin D levels of the subjects were examined by immunoblotting and enzyme-linked immunosorbent assay (ELISA). Correlation of plasma cathepsin D levels with AD-related factors and clinical characteristics were examined by statistical analysis. By analyzing quantitative immunoblot and ELISA, we found that the plasma level of cathepsin D, a major lysosomal protease, was decreased in the group with amyloid plaque deposition at the brain compared to the control group. The level of plasma cathepsin D was negatively correlated with clinical dementia rating scale sum of boxes (CDR-SB) scores. In addition, our integrated multivariable logistic regression model suggests the high performance of plasma cathepsin D level for discriminating AD from non-AD. These results suggest that the plasma cathepsin D level could be developed as a diagnostic biomarker candidate for AD.
Full article
►▼
Show Figures
Open AccessArticle
Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale
by
Nicolas Ruffini, Susanne Klingenberg, Susann Schweiger and Susanne Gerber
Cited by 29 | Viewed by 4979
Abstract
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) are heterogeneous, progressive diseases with frequently overlapping symptoms characterized by a loss of neurons. Studies have suggested relations between neurodegenerative diseases for many years (e.g.,
[...] Read more.
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) are heterogeneous, progressive diseases with frequently overlapping symptoms characterized by a loss of neurons. Studies have suggested relations between neurodegenerative diseases for many years (e.g., regarding the aggregation of toxic proteins or triggering endogenous cell death pathways). We gathered publicly available genomic, transcriptomic, and proteomic data from 177 studies and more than one million patients to detect shared genetic patterns between the neurodegenerative diseases on three analyzed omics-layers. The results show a remarkably high number of shared differentially expressed genes between the transcriptomic and proteomic levels for all conditions, while showing a significant relation between genomic and proteomic data between AD and PD and AD and ALS. We identified a set of 139 genes being differentially expressed in several transcriptomic experiments of all four diseases. These 139 genes showed overrepresented gene ontology (GO) Terms involved in the development of neurodegeneration, such as response to heat and hypoxia, positive regulation of cytokines and angiogenesis, and RNA catabolic process. Furthermore, the four analyzed neurodegenerative diseases (NDDs) were clustered by their mean direction of regulation throughout all transcriptomic studies for this set of 139 genes, with the closest relation regarding this common gene set seen between AD and HD. GO-Term and pathway analysis of the proteomic overlap led to biological processes (BPs), related to protein folding and humoral immune response. Taken together, we could confirm the existence of many relations between Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis on transcriptomic and proteomic levels by analyzing the pathways and GO-Terms arising in these intersections. The significance of the connection and the striking relation of the results to processes leading to neurodegeneration between the transcriptomic and proteomic data for all four analyzed neurodegenerative diseases showed that exploring many studies simultaneously, including multiple omics-layers of different neurodegenerative diseases simultaneously, holds new relevant insights that do not emerge from analyzing these data separately. Furthermore, the results shed light on processes like the humoral immune response that have previously been described only for certain diseases. Our data therefore suggest human patients with neurodegenerative diseases should be addressed as complex biological systems by integrating multiple underlying data sources.
Full article
►▼
Show Figures