Molecular Determinants of Skin Integrity
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 26154
Share This Topical Collection
Editor
 Dr. Michèle T. Martin
Dr. Michèle T. Martin
 Dr. Michèle T. Martin
Dr. Michèle T. Martin
E-Mail
Website
Collection Editor
1. Laboratoire de Génomique et Radiobiologie de la Kératinopoïèse, Institut de Biologie François Jacob, CEA/DRF, Institut de Radiobiologie Cellulaire et Moléculaire, 91000 Evry, France
2. Université Paris-Saclay, 91190 Saint-Aubin, France
Interests: human skin biology and pathology; stem cells; genomic stability; TGFB1; KLF4; SHH
Topical Collection Information
Dear Colleagues,
Skin is the first body defense against external stress. In the epidermis, keratinocyte stem cells are responsible for monthly physiological replacement and for regeneration during wound healing, and differentiated layers ensure the skin barrier function. In the dermis, various cell types ensure the maintenance of tissue structures and functionalities, notably the fibroblasts through constant ECM synthesis and remodeling, and immune cells that coordinate local and general regulations. Skin integrity is lost in various pathological conditions, including genotoxic stress, burns, chronic inflammation, and ulcers, which disrupt the complex mechanisms regulating skin homeostasis. Identification of the perturbated molecular mechanisms is key to obtain in-depth knowledge on pathological contexts, and requires dissecting perturbed cellular interactions and abnormal microenvironments. Such fundamental research can help in identifying targets or molecules of interest for skin care and repair, and redirect this tissue towards homeostasis.
Advances in preservation and repair of skin integrity can benefit from research and preclinical models, including organoid formation and reconstitution through skin substitutes, exposome assays, as well as the analysis of genome, transcriptome, and epigenome through NGS and single-cell analysis in native and gene-edited cells.
In this Topical Collection of Cells, we will gather articles and reviews on recent fundamental and applied advances in skin integrity. The impact of epigenomic controls of cell function and stability will be particularly welcome.
Dr. Michèle T. Martin
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (8 papers)
Open AccessArticle
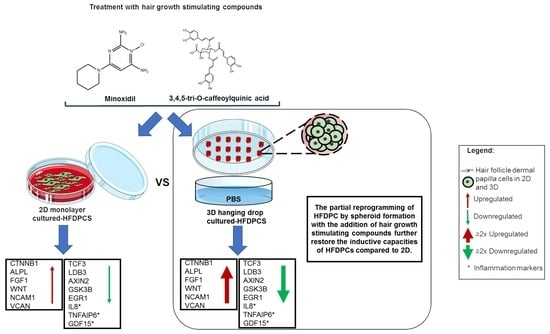
3D Spheroid Human Dermal Papilla Cell as an Effective Model for the Screening of Hair Growth Promoting Compounds: Examples of Minoxidil and 3,4,5-Tri-O-caffeoylquinic acid (TCQA)
by
Meriem Bejaoui, Aprill Kee Oliva, May Sin Ke, Farhana Ferdousi and Hiroko Isoda
Cited by 6 | Viewed by 3871
Abstract
Dermal papilla cells (DPCs) are an important element of the hair follicle (HF) niche, widely used as an in vitro model to study hair growth-related research. These cells are usually grown in 2D culture, but this system did not show efficient therapeutic effects
[...] Read more.
Dermal papilla cells (DPCs) are an important element of the hair follicle (HF) niche, widely used as an in vitro model to study hair growth-related research. These cells are usually grown in 2D culture, but this system did not show efficient therapeutic effects on HF regeneration and growth, and key differences were observed between cell activity in vitro and in vivo. Recent studies have showed that DPCs grown in 3D hanging spheroids are more morphologically akin to an intact DP microenvironment. In this current study, global gene molecular analysis showed that the 3D model highly affected cell adhesion molecules and hair growth-related pathways. Furthermore, we compared the expression of signalling molecules and metabolism-associated proteins of DPCs treated with minoxidil (an FDA-approved drug for hair loss treatment) and 3,4,5-tri-
O-caffeoylquinic acid (TCQA) (recently found to induce hair growth in vitro and in vivo) in 3D spheroid hanging drops and a 2D monolayer using DNA microarray analysis. Further validations by determining the gene and protein expressions of key signature molecules showed the suitability of this 3D system for enhancing the DPC activity of the hair growth-promoting agents minoxidil and TCQA.
Full article
►▼
Show Figures
Open AccessArticle
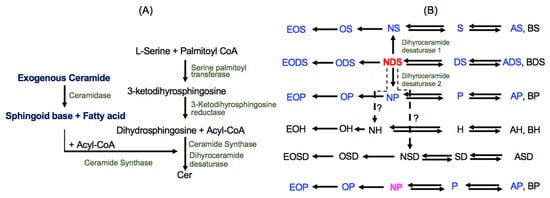
Exogenous Ceramide Serves as a Precursor to Endogenous Ceramide Synthesis and as a Modulator of Keratinocyte Differentiation
by
Kyong-Oh Shin, Hisashi Mihara, Kenya Ishida, Yoshikazu Uchida and Kyungho Park
Cited by 5 | Viewed by 2462
Abstract
Since ceramide is a key epidermal barrier constituent and its deficiency causes barrier-compromised skin, several molecular types of ceramides are formulated in commercial topical agents to improve barrier function. Topical ceramide localizes on the skin surface and in the stratum corneum, but certain
[...] Read more.
Since ceramide is a key epidermal barrier constituent and its deficiency causes barrier-compromised skin, several molecular types of ceramides are formulated in commercial topical agents to improve barrier function. Topical ceramide localizes on the skin surface and in the stratum corneum, but certain amounts of ceramide penetrate the stratum granulosum, becoming precursors to endogenous ceramide synthesis following molecular modification. Moreover, exogenous ceramide as a lipid mediator could modulate keratinocyte proliferation/differentiation. We here investigated the biological roles of exogenous NP (non-hydroxy ceramide containing 4-hydroxy dihydrosphingosine) and NDS (non-hydroxy ceramide containing dihydrosphingosine), both widely used as topical ceramide agents, in differentiated-cultured human keratinocytes. NDS, but not NP, becomes a precursor for diverse ceramide species that are required for a vital permeability barrier. Loricrin (late differentiation marker) production is increased in keratinocytes treated with both NDS and NP vs. control, while bigger increases in involucrin (an early differentiation marker) synthesis were observed in keratinocytes treated with NDS vs. NP and control. NDS increases levels of a key antimicrobial peptide (an innate immune component), cathelicidin antimicrobial peptide (CAMP/LL-37), that is upregulated by a ceramide metabolite, sphingosine-1-phosphate. Our studies demonstrate that NDS could be a multi-potent ceramide species, forming heterogenous ceramide molecules and a lipid mediator to enhance differentiation and innate immunity.
Full article
►▼
Show Figures
Open AccessArticle
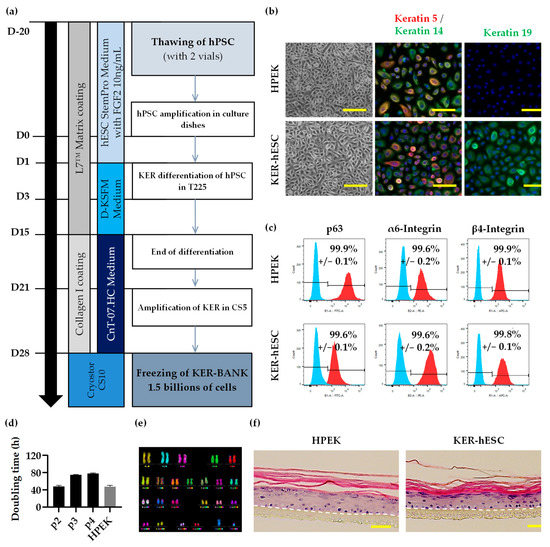
Clinical Grade Human Pluripotent Stem Cell-Derived Engineered Skin Substitutes Promote Keratinocytes Wound Closure In Vitro
by
Sophie Domingues, Annabelle Darle, Yolande Masson, Manoubia Saidani, Emilie Lagoutte, Ana Bejanariu, Julien Coutier, Raif Eren Ayata, Marielle Bouschbacher, Marc Peschanski, Gilles Lemaitre and Christine Baldeschi
Cited by 1 | Viewed by 2475
Abstract
Chronic wounds, such as leg ulcers associated with sickle cell disease, occur as a consequence of a prolonged inflammatory phase during the healing process. They are extremely hard to heal and persist as a significant health care problem due to the absence of
[...] Read more.
Chronic wounds, such as leg ulcers associated with sickle cell disease, occur as a consequence of a prolonged inflammatory phase during the healing process. They are extremely hard to heal and persist as a significant health care problem due to the absence of effective treatment and the uprising number of patients. Indeed, there is a critical need to develop novel cell- and tissue-based therapies to treat these chronic wounds. Development in skin engineering leads to a small catalogue of available substitutes manufactured in Good Manufacturing Practices compliant (GMPc) conditions. Those substitutes are produced using primary cells that could limit their use due to restricted sourcing. Here, we propose GMPc protocols to produce functional populations of keratinocytes and fibroblasts derived from pluripotent stem cells to reconstruct the associated dermo-epidermal substitute with plasma-based fibrin matrix. In addition, this manufactured composite skin is biologically active and enhances in vitro wounding of keratinocytes. The proposed composite skin opens new perspectives for skin replacement using allogeneic substitute.
Full article
►▼
Show Figures
Open AccessArticle
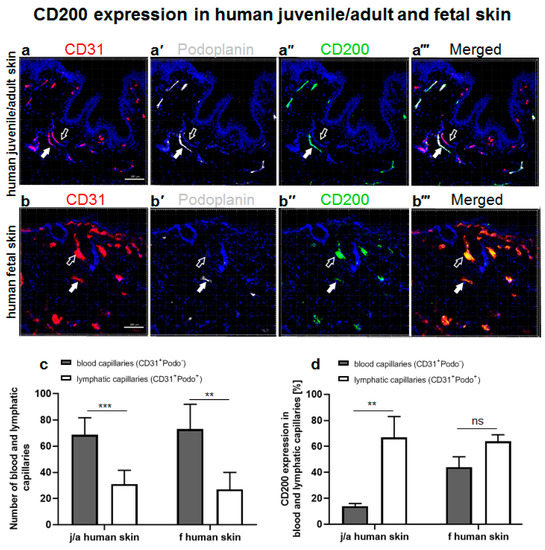
The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation
by
Dominic Rütsche, Katarzyna Michalak-Micka, Dominika Zielinska, Hannah Moll, Ueli Moehrlen, Thomas Biedermann and Agnes S. Klar
Cited by 5 | Viewed by 3317
Abstract
CD200 is a cell membrane glycoprotein that interacts with its structurally related receptor (CD200R) expressed on immune cells. We characterized CD200–CD200R interactions in human adult/juvenile (j/a) and fetal (f) skin and in in vivo prevascularized skin substitutes (vascDESS) prepared by co-culturing human dermal
[...] Read more.
CD200 is a cell membrane glycoprotein that interacts with its structurally related receptor (CD200R) expressed on immune cells. We characterized CD200–CD200R interactions in human adult/juvenile (j/a) and fetal (f) skin and in in vivo prevascularized skin substitutes (vascDESS) prepared by co-culturing human dermal microvascular endothelial cells (HDMEC), containing both blood (BEC) and lymphatic (LEC) EC. We detected the highest expression of CD200 on lymphatic capillaries in j/a and f skin as well as in vascDESS in vivo, whereas it was only weakly expressed on blood capillaries. Notably, the highest CD200 levels were detected on LEC with enhanced Podoplanin expression, while reduced expression was observed on Podoplanin-low LEC. Further, qRT-PCR analysis revealed upregulated expression of some chemokines, including CC-chemokine ligand 21 (CCL21) in j/aCD200
+ LEC, as compared to j/aCD200
− LEC. The expression of CD200R was mainly detected on myeloid cells such as granulocytes, monocytes/macrophages, T cells in human peripheral blood, and human and rat skin. Functional immunoassays demonstrated specific binding of skin-derived CD200
+ HDMEC to myeloid CD200R
+ cells in vitro. Importantly, we confirmed enhanced CD200–CD200R interaction in vascDESS in vivo. We concluded that the CD200–CD200R axis plays a crucial role in regulating tissue inflammation during skin wound healing.
Full article
►▼
Show Figures
Open AccessArticle
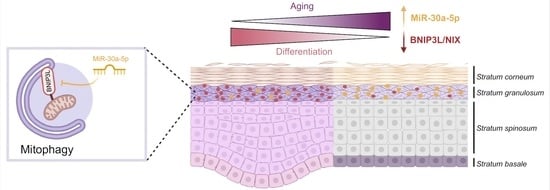
MiR-30a-5p Alters Epidermal Terminal Differentiation during Aging by Regulating BNIP3L/NIX-Dependent Mitophagy
by
Fabien P. Chevalier, Julie Rorteau, Sandra Ferraro, Lisa S. Martin, Alejandro Gonzalez-Torres, Aurore Berthier, Naima El Kholti and Jérôme Lamartine
Cited by 7 | Viewed by 2943
Abstract
Chronological aging is characterized by an alteration in the genes’ regulatory network. In human skin, epidermal keratinocytes fail to differentiate properly with aging, leading to the weakening of the epidermal function. MiR-30a is particularly overexpressed with epidermal aging, but the downstream molecular mechanisms
[...] Read more.
Chronological aging is characterized by an alteration in the genes’ regulatory network. In human skin, epidermal keratinocytes fail to differentiate properly with aging, leading to the weakening of the epidermal function. MiR-30a is particularly overexpressed with epidermal aging, but the downstream molecular mechanisms are still uncovered. The aim of this study was to decipher the effects of miR-30a overexpression in the human epidermis, with a focus on keratinocyte differentiation. We formally identified the mitophagy receptor BNIP3L as a direct target of miR-30a. Using a 3D organotypic model of reconstructed human epidermis overexpressing miR-30a, we observed a strong reduction in BNIP3L expression in the granular layer. In human epidermal sections of skin biopsies from donors of different ages, we observed a similar pattern of BNIP3L decreasing with aging. Moreover, human primary keratinocytes undergoing differentiation in vitro also showed a decreased expression of
BNIP3L with age, together with a retention of mitochondria. Moreover, aging is associated with altered mitochondrial metabolism in primary keratinocytes, including decreased ATP-linked respiration. Thus, miR-30a is a negative regulator of programmed mitophagy during keratinocytes terminal differentiation, impairing epidermal homeostasis with aging.
Full article
►▼
Show Figures
Open AccessArticle
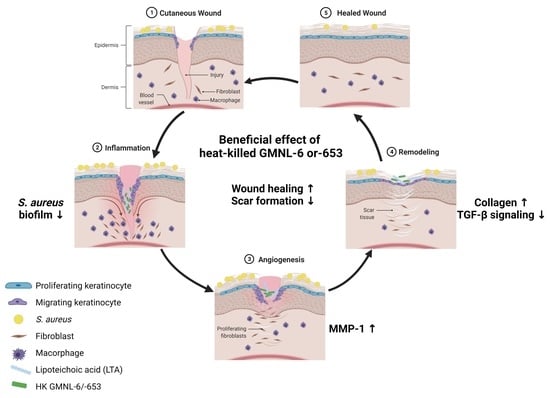
Heat-Killed Lactobacilli Preparations Promote Healing in the Experimental Cutaneous Wounds
by
Wan-Hua Tsai, Chia-Hsuan Chou, Tsuei-Yin Huang, Hui-Ling Wang, Peng-Ju Chien, Wen-Wei Chang and Hsueh-Te Lee
Cited by 14 | Viewed by 3269
Abstract
Probiotics are defined as microorganisms with beneficial health effects when consumed by humans, being applied mainly to improve allergic or intestinal diseases. Due to the increasing resistance of pathogens to antibiotics, the abuse of antibiotics becomes inefficient in the skin and in systemic
[...] Read more.
Probiotics are defined as microorganisms with beneficial health effects when consumed by humans, being applied mainly to improve allergic or intestinal diseases. Due to the increasing resistance of pathogens to antibiotics, the abuse of antibiotics becomes inefficient in the skin and in systemic infections, and probiotics may also provide the protective effect for repairing the healing of infected cutaneous wounds. Here we selected two Lactobacillus strains,
L. plantarum GMNL-6 and
L. paracasei GMNL-653, in heat-killed format to examine the beneficial effect in skin wound repair through the selection by promoting collagen synthesis in Hs68 fibroblast cells. The coverage of gels containing heat-killed GMNL-6 or GMNL-653 on the mouse tail with experimental wounds displayed healing promoting effects with promoting of metalloproteinase-1 expression at the early phase and reduced excessive fibrosis accumulation and deposition in the later tail-skin recovery stage. More importantly, lipoteichoic acid, the major component of Lactobacillus cell wall, from GMNL-6/GMNL-653 could achieve the anti-fibrogenic benefit similar to the heat-killed bacteria cells in the TGF-β stimulated Hs68 fibroblast cell model. Our study offers a new therapeutic potential of the heat-killed format of Lactobacillus as an alternative approach to treating skin healing disorders.
Full article
►▼
Show Figures
Open AccessReview
Epidermal Hyaluronan in Barrier Alteration-Related Disease
by
Céline Evrard, Catherine Lambert de Rouvroit and Yves Poumay
Cited by 9 | Viewed by 2889
Abstract
In skin, although the extracellular matrix (ECM) is highly developed in dermis and hypodermis, discrete intercellular spaces between cells of the living epidermal layers are also filled with ECM components. Herein, we review knowledge about structure, localization and role of epidermal hyaluronan (HA),
[...] Read more.
In skin, although the extracellular matrix (ECM) is highly developed in dermis and hypodermis, discrete intercellular spaces between cells of the living epidermal layers are also filled with ECM components. Herein, we review knowledge about structure, localization and role of epidermal hyaluronan (HA), a key ECM molecule. HA is a non-sulfated glycosaminoglycan non-covalently bound to proteins or lipids. Components of the basal lamina maintain some segregation between the epidermis and the underlying dermis, and all epidermal HA is locally synthesized and degraded. Functions of HA in keratinocyte proliferation and differentiation are still controversial. However, through interactions with partners, such as the TSG-6 protein, HA is involved in the formation, organization and stabilization of the epidermal ECM. In addition, epidermal HA is involved in the formation of an efficient epidermal barrier made of cornified keratinocytes. In atopic dermatitis (AD) with profuse alterations of the epidermal barrier, HA is produced in larger amounts by keratinocytes than in normal skin. Epidermal HA inside AD lesional skin is located in enlarged intercellular spaces, likely as the result of disease-related modifications of HA metabolism.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
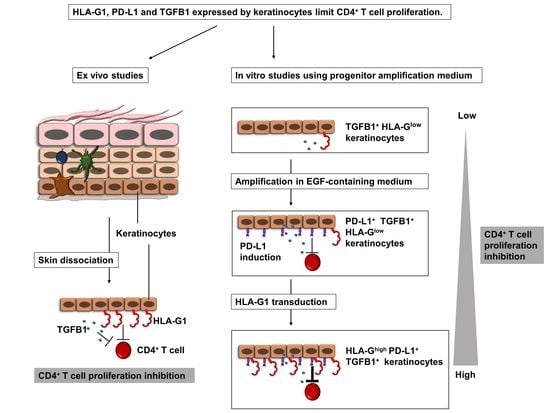
Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints
by
Guillaume Mestrallet, Frédéric Auvré, Chantal Schenowitz, Edgardo D. Carosella, Joel LeMaoult, Michèle T. Martin, Nathalie Rouas-Freiss and Nicolas O. Fortunel
Cited by 8 | Viewed by 3818
Abstract
Human skin protects the body against infection and injury. This protection involves immune and epithelial cells, but their interactions remain largely unknown. Here, we show that cultured epidermal keratinocytes inhibit allogenic CD4
+ T-cell proliferation under both normal and inflammatory conditions. Inhibition occurs
[...] Read more.
Human skin protects the body against infection and injury. This protection involves immune and epithelial cells, but their interactions remain largely unknown. Here, we show that cultured epidermal keratinocytes inhibit allogenic CD4
+ T-cell proliferation under both normal and inflammatory conditions. Inhibition occurs through the secretion of soluble factors, including TGFB1 and the cell-surface expression of HLA-G1 and PD-L1 immune checkpoints. For the first time, we here describe the expression of the HLA-G1 protein in healthy human skin and its role in keratinocyte-driven tissue immunomodulation. The overexpression of HLA-G1 with an inducible vector increased the immunosuppressive properties of keratinocytes, opening up perspectives for their use in allogeneic settings for cell therapy.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: MiR-30a-5p alters epidermal differentiation by regulating BNIP3L/NIX-dependent mitophagy
Authors: Fabien CHEVALIER; et.al
Affiliation: Maître de Conférences des Universités Lyon 1 University Laboratory of Tissue Biology and Therapeutic Engineering Team Skin function and dynamics 7 Passage du Vercors 69367 LYON Cedex 07 FRANCE
Abstract: /
Title: Epigenetic regulation of wound healing
Authors: Maurizio Romagnuolo; Chiara Moltrasio
Affiliation: Resident in Dermatology and Venereology
Department of Pathophysiology and Transplantation
Foundation IRCCS Ca\' Granda Ospedale Maggiore Policlinico, Milan, Italy.
Abstract: Regulation of the wound healing process involves multiple cell types and regulatory pathways as well as several epigenetic mechanisms of gene regulation, such as DNA methylation, histone modifications, chromatin remodeling, and non-coding RNAs. Epigenetic regulatory mechanisms are mandatory for epidermal homeostasis and integrity and, when altered, contribute to the pathogenesis of several cutaneous disorders, including skin cancer and inflammatory skin diseases like psoriasis, hidradenitis suppurativa and pyoderma gangrenosum.
Epigenetic regulators appear to be involved in the processes of skin repair, modulating keratinocyte proliferation, differentiation, and migration, together with dermal regeneration and neo angiogenesis; all this is made possible by the dynamic and continuous interaction with specific mediators able to both stimulate and repress gene activation, transiently altering cellular phenotype and behaviour.
Understanding the molecular basis of epigenetic regulation is necessary to discover new potential therapeutic targets for the treatment of both acute and chronic skin diseases.
Here we will discuss the epigenetic changes that contribute to wound healing by specific cell type, and we will highlight the role of epigenetic regulators in the molecular pathophysiology of chronic wound conditions.