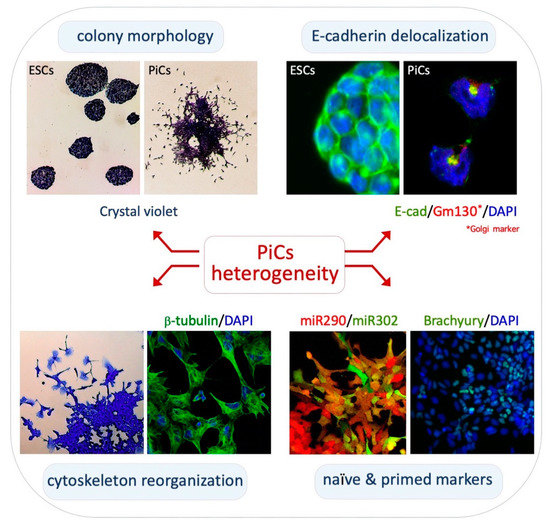
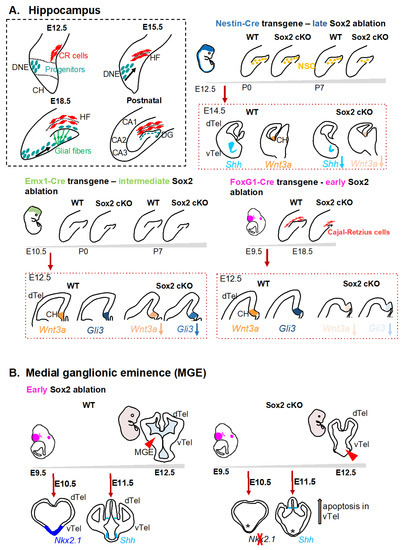
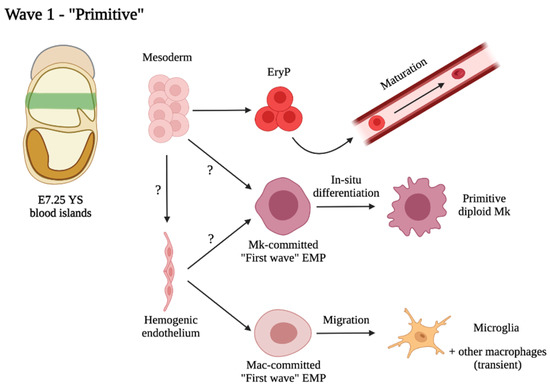
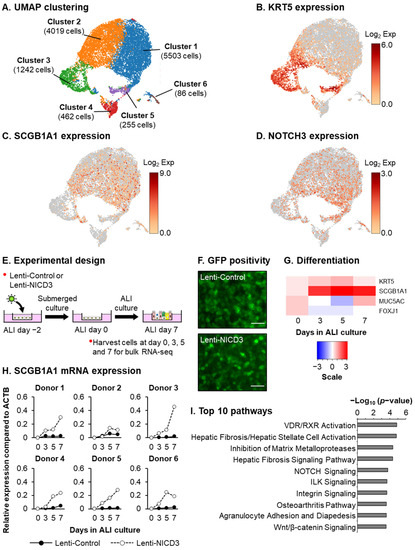
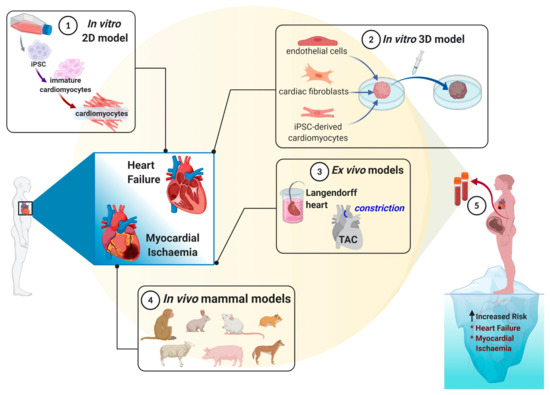
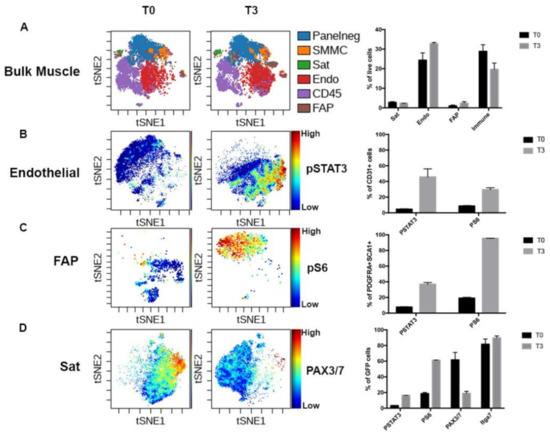
Molecular Basis of Cellular Heterogeneity in Physiology and Disease: Diversity Matters
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 51627Editors
Interests: cell plasticity; stem cells; skeletal muscle regeneration; Cripto
Interests: stem cells; pluripotency/differentiation; neuroscience; neurodegenerative disease; cell metabolism; cell signaling; non-coding RNA
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection of Cells welcomes original articles and reviews covering the broad spectrum of processes in which cellular heterogeneity and plasticity are involved both in health and disease, with a preferential focus on stem cells. In addition to the concept that heterogeneity is inherent to stem cell populations, increasing evidence supports the idea that stem cells are dynamically heterogeneous, being able to fluctuate between different phenotypic and functional states endowed with distinct differentiation/fate potential. Such plasticity is important for embryonic development, to maintain tissue homeostasis, and for tissue regeneration. However, cell plasticity is not confined to physiological processes but is reactivated at the onset of several pathological conditions, including cancer. Indeed, targeting cellular heterogeneity is still a major challenge in the effective treatment of cancer.
Uncovering the molecular basis of cellular heterogeneity is thus relevant both for understanding developmental mechanisms and for anticancer therapies.
Dr. Gabriella Minchiotti
Dr. Annalisa Fico
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cell plasticity
- stem cells
- tissue regeneration
- cancer