MicroRNAs in Cancer: From Molecular Mechanisms to Applications in Diagnostic and Therapeutic Opportunities
Share This Topical Collection
Editor
 Dr. Tohru Yamada
Dr. Tohru Yamada
 Dr. Tohru Yamada
Dr. Tohru Yamada
E-Mail
Website
Collection Editor
Department of Surgery, Division of Surgical Oncology, University of Illinois, Chicago, IL, USA
Interests: brain tumors; molecular biology; protein/peptide chemistry; cell biology; basic and translational cancer biology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
MicroRNAs (miRNAs) are small noncoding RNAs that play important roles in gene regulation by repressing translation or directing sequence-specific degradation of complementary mRNA or inducing gene expression via binding to promoter sequences, therefore regulating cell growth and development. MiRNAs are also known to regulate tumor progression. miRNAs have gained significant attention over the last decade due to their great potential in the development of the next-generation approaches to fight against cancer.
This Topical Collection of Cells invites investigators to contribute original research articles, reviews, and perspectives focusing on recent advances in cancer research with miRNAs (e.g., therapy and biomarkers for diagnosis). Manuscripts solely describing bioinformatics or in silico computational analysis of public databases that are not accompanied by biological validation will not be considered. We are looking for contributions that primarily focus on the use of miRNAs for various types of cancer, including rarer varieties.
Dr. Tohru Yamada
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- oncogenes
- tumor suppressors
- circulating miRNA
- targeting delivery
- chemical modifications
- cargo molecule conjugations
Published Papers (5 papers)
Open AccessArticle
Clinical Relevance and Interplay between miRNAs in Influencing Glioblastoma Multiforme Prognosis
by
Samantha Epistolio, Giulia Dazio, Ismail Zaed, Nora Sahnane, Debora Cipriani, Francesco Polinelli, Jessica Barizzi, Paolo Spina, Federico Mattia Stefanini, Michele Cerati, Sergio Balbi, Luca Mazzucchelli, Fausto Sessa, Gianfranco Angelo Pesce, Michael Reinert, Andrea Cardia, Francesco Marchi and Milo Frattini
Viewed by 756
Abstract
Glioblastoma multiforme (GBM) is usually treated with surgery followed by adjuvant partial radiotherapy combined with temozolomide (TMZ) chemotherapy. Recent studies demonstrated a better survival and good response to TMZ in methylguanine-DNA methyltransferase (
MGMT)-methylated GBM cases. However, approximately 20% of patients with
[...] Read more.
Glioblastoma multiforme (GBM) is usually treated with surgery followed by adjuvant partial radiotherapy combined with temozolomide (TMZ) chemotherapy. Recent studies demonstrated a better survival and good response to TMZ in methylguanine-DNA methyltransferase (
MGMT)-methylated GBM cases. However, approximately 20% of patients with
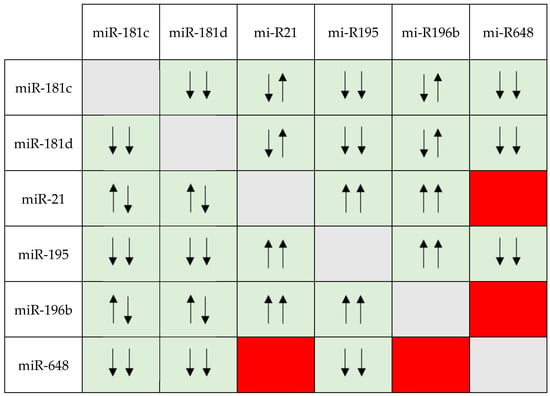
MGMT-unmethylated GBM display an unexpectedly favorable outcome. Therefore, additional mechanisms related to the TMZ response need to be investigated. As such, we decided to investigate the clinical relevance of six miRNAs involved in brain tumorigenesis (miR-181c, miR-181d, miR-21, miR-195, miR-196b, miR-648) as additional markers of response and survival in patients receiving TMZ for GBM. We evaluated miRNA expression and the interplay between miRNAs in 112
IDH wt GBMs by applying commercial assays. Then, we correlated the miRNA expression with patients’ clinical outcomes. Upon bivariate analyses, we found a significant association between the expression levels of the miRNAs analyzed, but, more interestingly, the OS curves show that the combination of low miR-648 and miR-181c or miR-181d expressions is associated with a worse prognosis than cases with other low-expression miRNA pairs. To conclude, we found how specific miRNA pairs can influence survival in GBM cases treated with TMZ.
Full article
►▼
Show Figures
Open AccessArticle
The Sensitization of Triple-Negative Breast Cancers to Poly ADP Ribose Polymerase Inhibition Independent of BRCA1/2 Mutation Status by Chemically Modified microRNA-489
by
Young Hwa Soung, Jingfang Ju and Jun Chung
Viewed by 916
Abstract
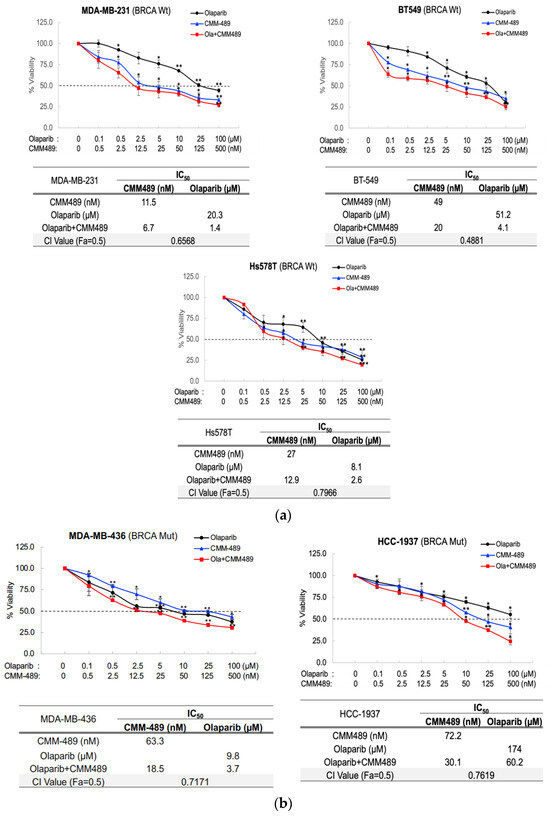
Chemoresistance and inefficient therapeutic efficacies in triple-negative breast cancers (TNBCs) are among the major clinical problems in breast cancers. A potential new method to sensitize these tumors to current treatment options is, therefore, urgent and necessary. Our previous studies demonstrated that miR-489 serves
[...] Read more.
Chemoresistance and inefficient therapeutic efficacies in triple-negative breast cancers (TNBCs) are among the major clinical problems in breast cancers. A potential new method to sensitize these tumors to current treatment options is, therefore, urgent and necessary. Our previous studies demonstrated that miR-489 serves as one of the top tumor-suppressing miRs and features downregulated expression in metastatic TNBCs and that the restoration of miR-489 expression in TNBCs effectively inhibits the metastatic potentials of TNBCs both in vitro and in vivo. The chemical modification of miR-489 (CMM489) through the replacement of uracil with 5-FU further enhances the therapeutic potential of miR-489. In the present study, we tested the effects of CMM489 in synergizing DNA damage response (DDR) inhibitors such as PARP inhibitors. CMM489 is particularly effective in sensitizing TNBC cell lines with inherent resistance to PARP inhibitors regardless of BRCA mutation status. One of the anti-cancer mechanisms through which CMM489 synergizes with PARP inhibitors is the blockade of homologous recombination (HR) in TNBC cells upon DNA damage. The results of this study highlight the potential use of CMM489 in combination treatments with PARP inhibitors in TNBCs.
Full article
►▼
Show Figures
Open AccessArticle
MicroRNA-Based Discovery of Biomarkers, Therapeutic Targets, and Repositioning Drugs for Breast Cancer
by
Qing Ye, Rebecca A. Raese, Dajie Luo, Juan Feng, Wenjun Xin, Chunlin Dong, Yong Qian and Nancy Lan Guo
Cited by 5 | Viewed by 1746
Abstract
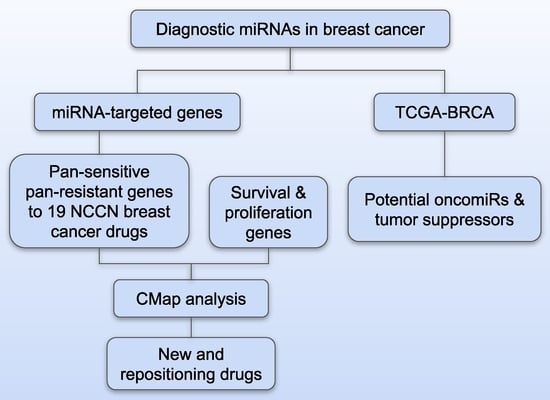
Breast cancer treatment can be improved with biomarkers for early detection and individualized therapy. A set of 86 microRNAs (miRNAs) were identified to separate breast cancer tumors from normal breast tissues (
n = 52) with an overall accuracy of 90.4%. Six miRNAs
[...] Read more.
Breast cancer treatment can be improved with biomarkers for early detection and individualized therapy. A set of 86 microRNAs (miRNAs) were identified to separate breast cancer tumors from normal breast tissues (
n = 52) with an overall accuracy of 90.4%. Six miRNAs had concordant expression in both tumors and breast cancer patient blood samples compared with the normal control samples. Twelve miRNAs showed concordant expression in tumors vs. normal breast tissues and patient survival (
n = 1093), with seven as potential tumor suppressors and five as potential oncomiRs. From experimentally validated target genes of these 86 miRNAs, pan-sensitive and pan-resistant genes with concordant mRNA and protein expression associated with in-vitro drug response to 19 NCCN-recommended breast cancer drugs were selected. Combined with in-vitro proliferation assays using CRISPR-Cas9/RNAi and patient survival analysis, MEK inhibitors PD19830 and BRD-K12244279, pilocarpine, and tremorine were discovered as potential new drug options for treating breast cancer. Multi-omics biomarkers of response to the discovered drugs were identified using human breast cancer cell lines. This study presented an artificial intelligence pipeline of miRNA-based discovery of biomarkers, therapeutic targets, and repositioning drugs that can be applied to many cancer types.
Full article
►▼
Show Figures
Open AccessReview
MicroRNAs in Tumor Endothelial Cells: Regulation, Function and Therapeutic Applications
by
Yuan Gu, Maximilian A. Becker, Luisa Müller, Katharina Reuss, Frederik Umlauf, Tianci Tang, Michael D. Menger and Matthias W. Laschke
Cited by 2 | Viewed by 1623
Abstract
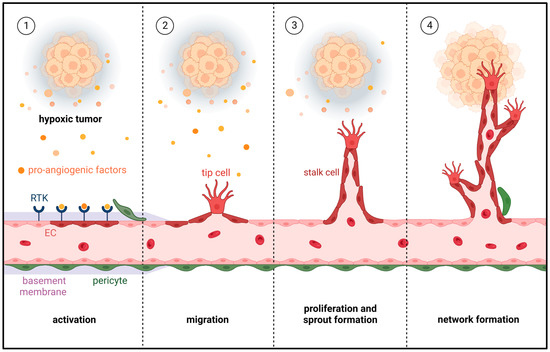
Tumor endothelial cells (TECs) are key stromal components of the tumor microenvironment, and are essential for tumor angiogenesis, growth and metastasis. Accumulating evidence has shown that small single-stranded non-coding microRNAs (miRNAs) act as powerful endogenous regulators of TEC function and blood vessel formation.
[...] Read more.
Tumor endothelial cells (TECs) are key stromal components of the tumor microenvironment, and are essential for tumor angiogenesis, growth and metastasis. Accumulating evidence has shown that small single-stranded non-coding microRNAs (miRNAs) act as powerful endogenous regulators of TEC function and blood vessel formation. This systematic review provides an up-to-date overview of these endothelial miRNAs. Their expression is mainly regulated by hypoxia, pro-angiogenic factors, gap junctions and extracellular vesicles, as well as long non-coding RNAs and circular RNAs. In preclinical studies, they have been shown to modulate diverse fundamental angiogenesis-related signaling pathways and proteins, including the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) pathway; the rat sarcoma virus (Ras)/rapidly accelerated fibrosarcoma (Raf)/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway; the phosphoinositide 3-kinase (PI3K)/AKT pathway; and the transforming growth factor (TGF)-β/TGF-β receptor (TGFBR) pathway, as well as krüppel-like factors (KLFs), suppressor of cytokine signaling (SOCS) and metalloproteinases (MMPs). Accordingly, endothelial miRNAs represent promising targets for future anti-angiogenic cancer therapy. To achieve this, it will be necessary to further unravel the regulatory and functional networks of endothelial miRNAs and to develop safe and efficient TEC-specific miRNA delivery technologies.
Full article
►▼
Show Figures
Open AccessReview
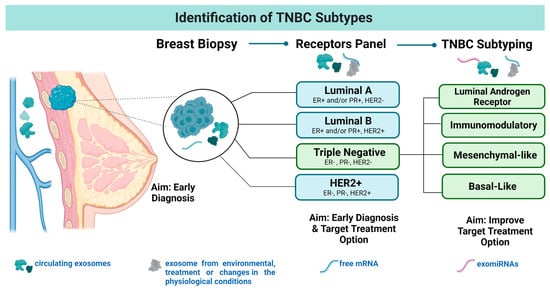
Identification of Exo-miRNAs: A Summary of the Efforts in Translational Studies Involving Triple-Negative Breast Cancer
by
Jarline Encarnación-Medina, Lenin Godoy, Jaime Matta and Carmen Ortiz-Sánchez
Cited by 2 | Viewed by 1749
Abstract
Triple-negative breast cancer (TNBC) accounts for about 10–15% of all breast cancers (BC) in the US and its diagnosis is associated with poor survival outcomes. A better understanding of the disease etiology is crucial to identify target treatment options to improve patient outcomes.
[...] Read more.
Triple-negative breast cancer (TNBC) accounts for about 10–15% of all breast cancers (BC) in the US and its diagnosis is associated with poor survival outcomes. A better understanding of the disease etiology is crucial to identify target treatment options to improve patient outcomes. The role of exo-miRNAs in TNBC has been studied for more than two decades. Although some studies have identified exo-miR candidates in TNBC using clinical samples, consensus regarding exo-miR candidates has not been achieved. The purpose of this review is to gather information regarding exo-miR candidates reported in TNBC translational studies along with the techniques used to isolate and validate the potential targets. The techniques suggested in this review are based on the use of commercially available materials for research and clinical laboratories. We expect that the information included in this review can add additional value to the recent efforts in the development of a liquid biopsy to identify TNBC cases and further improve their survival outcomes.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: miR181 - a much ignored miR in HCC
Authors: Geoff McCaughan
Affiliation: University of Sydney, Sydney, Australia
Title: micro-RNAs as diagnostic tool of colon cancer
Authors: Younghwa Song
Affiliation: Stony Brook Medicine, Stony Brook, NY, USA
Title: MicroRNAs in peripheral T-cell Lymphoma pathogenesis
Authors: Dylan Jochum, Javeed Iqbal et al
Affiliation: University of Nebraska Medical Center, Omaha, NE, USA
Title: MicroRNA-122 inhibits colon cancer cell proliferation and migration by directly regulating MTDH protein
Authors: Surajit Pathak; Xiao Feng Sun
Affiliation: Linkoping University, Sweden