Advance in Ion Channel Signaling in Cancer Cells
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 22255
Share This Topical Collection
Editors
 Dr. Bruno Constantin
Dr. Bruno Constantin
 Dr. Bruno Constantin
Dr. Bruno Constantin
E-Mail
Website
Collection Editor
Laboratoire STIM, Université de Poitiers-CNRS ERL 7003, Poitiers, France
Interests: calcium channels; calcium signaling; stem cells; cancer stem cells; invasive cancer cells
 Prof. Dr. Valérie Coronas
Prof. Dr. Valérie Coronas
 Prof. Dr. Valérie Coronas
Prof. Dr. Valérie Coronas
E-Mail
Website
Collection Editor
Laboratoire STIM, Université de Poitiers-CNRS ERL 7003, Poitiers, France
Interests: calcium; stem cell; glioma; proliferation; microenvironment
Topical Collection Information
Dear Colleagues,
The large family of membrane-spanning ion channels contributes significantly to the transmission and integration of extracellular signals, both chemical and mechanical, that originate from the microenvironment and stroma where the cells reside. A growing body of studies points to a crucial role of ion channels in the dynamics of neoplastic cells. This collection will gather special contributions showing how cancer cells hijack the physiological functions of different types of ion channels and how changes in their activity and/or expression contribute to the neoplastic phenotype. This Topical Collection will also address the involvement of ion channels in a rare subpopulation of cells called cancer stem cells that possess the potential to initiate and sustain tumour growth and to regenerate the tumour after the cessation of treatment. This collection also aims to report the recent developments on the molecular mechanisms, e.g., ion transport or noncanonical channel functions, whereby ion channels contribute to various extents to the pathophysiological features of cancer hallmarks.
We look forward to your contribution in the form of original research or review articles.
Dr. Bruno Constantin
Prof. Dr. Valérie Coronas
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- ion channels
- cancers
- cancer stem cells
- invasive cancer cells
- ion channel crosstalk
- microenvironment
- mechanical stimulus transduction
Published Papers (7 papers)
Open AccessArticle
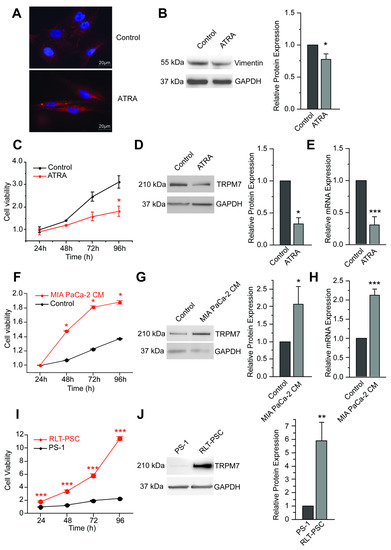
TRPM7 Modulates Human Pancreatic Stellate Cell Activation
by
Julie Auwercx, Philippe Kischel, Thibaut Lefebvre, Nicolas Jonckheere, Alison Vanlaeys, Stéphanie Guénin, Silviya Radoslavova, Isabelle Van Seuningen, Halima Ouadid-Ahidouch, Hemant M. Kocher, Isabelle Dhennin-Duthille and Mathieu Gautier
Cited by 4 | Viewed by 2195
Abstract
Pancreatic diseases, such as pancreatitis or pancreatic ductal adenocarcinoma, are characterized by the presence of activated pancreatic stellate cells (PSCs). These cells represent key actors in the tumor stroma, as they actively participate in disease development and progression: reprograming these PSCs into a
[...] Read more.
Pancreatic diseases, such as pancreatitis or pancreatic ductal adenocarcinoma, are characterized by the presence of activated pancreatic stellate cells (PSCs). These cells represent key actors in the tumor stroma, as they actively participate in disease development and progression: reprograming these PSCs into a quiescent phenotype has even been proposed as a promising strategy for restoring the hallmarks of a healthy pancreas. Since TRPM7 channels have been shown to regulate hepatic stellate cells proliferation and survival, we aimed to study the role of these magnesium channels in PSC activation and proliferation. PS-1 cells (isolated from a healthy pancreas) were used as a model of healthy PSCs: quiescence or activation were induced using all-trans retinoic acid or conditioned media of pancreatic cancer cells, respectively. The role of TRPM7 was studied by RNA silencing or by pharmacological inhibition. TRPM7 expression was found to be correlated with the activation status of PS-1 cells. TRPM7 expression was able to regulate proliferation through modulation of cell cycle regulators and most importantly p53, via the PI3K/Akt pathway, in a magnesium-dependent manner. Finally, the analysis of TCGA database showed the overexpression of TRPM7 in cancer-associated fibroblasts. Taken together, we provide strong evidences that TRPM7 can be considered as a marker of activated PSCs.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
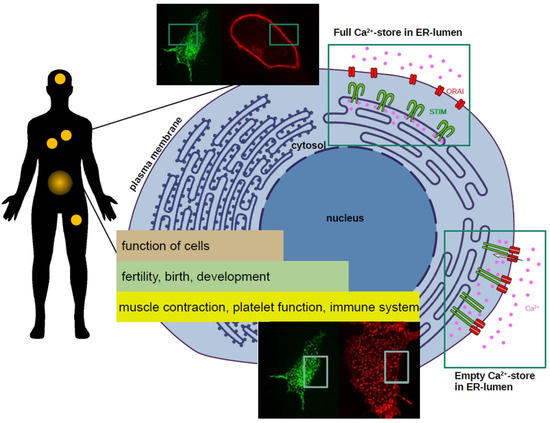
Science CommuniCa2+tion Developing Scientific Literacy on Calcium: The Involvement of CRAC Currents in Human Health and Disease
by
Christina Humer, Sascha Berlansky, Herwig Grabmayr, Matthias Sallinger, Andreas Bernhard, Marc Fahrner and Irene Frischauf
Cited by 3 | Viewed by 2252
Abstract
All human life starts with a calcium (Ca
2+) wave. This ion regulates a plethora of cellular functions ranging from fertilisation and birth to development and cell death. A sophisticated system is responsible for maintaining the essential, tight concentration of calcium within
[...] Read more.
All human life starts with a calcium (Ca
2+) wave. This ion regulates a plethora of cellular functions ranging from fertilisation and birth to development and cell death. A sophisticated system is responsible for maintaining the essential, tight concentration of calcium within cells. Intricate components of this Ca
2+ network are store-operated calcium channels in the cells’ membrane. The best-characterised store-operated channel is the Ca
2+ release-activated Ca
2+ (CRAC) channel. Currents through CRAC channels are critically dependent on the correct function of two proteins: STIM1 and Orai1. A disruption of the precise mechanism of Ca
2+ entry through CRAC channels can lead to defects and in turn to severe impacts on our health. Mutations in either STIM1 or Orai1 proteins can have consequences on our immune cells, the cardiac and nervous system, the hormonal balance, muscle function, and many more. There is solid evidence that altered Ca
2+ signalling through CRAC channels is involved in the hallmarks of cancer development: uncontrolled cell growth, resistance to cell death, migration, invasion, and metastasis. In this work we highlight the importance of Ca
2+ and its role in human health and disease with focus on CRAC channels.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
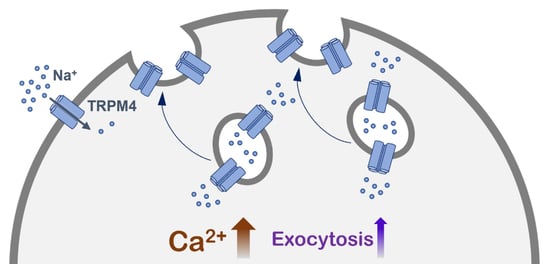
A Novel Role of the TRPM4 Ion Channel in Exocytosis
by
Paulina Stokłosa, Sven Kappel and Christine Peinelt
Cited by 7 | Viewed by 2201
Abstract
Under physiological conditions, the widely expressed calcium-activated TRPM4 channel conducts sodium into cells. This sodium influx depolarizes the plasma membrane and reduces the driving force for calcium entry. The aberrant expression or function of TRPM4 has been reported in various diseases, including different
[...] Read more.
Under physiological conditions, the widely expressed calcium-activated TRPM4 channel conducts sodium into cells. This sodium influx depolarizes the plasma membrane and reduces the driving force for calcium entry. The aberrant expression or function of TRPM4 has been reported in various diseases, including different types of cancer. TRPM4 is mainly localized in the plasma membrane, but it is also found in intracellular vesicles, which can undergo exocytosis. In this study, we show that calcium-induced exocytosis in the colorectal cancer cell line HCT116 is dependent on TRPM4. In addition, the findings from some studies of prostate cancer cell lines suggest a more general role of TRPM4 in calcium-induced exocytosis in cancer cells. Furthermore, calcium-induced exocytosis depends on TRPM4 ion conductivity. Additionally, an increase in intracellular calcium results in the delivery of TRPM4 to the plasma membrane. This process also depends on TRPM4 ion conductivity. TRPM4-dependent exocytosis and the delivery of TRPM4 to the plasma membrane are mediated by SNARE proteins. Finally, we provide evidence that calcium-induced exocytosis depends on TRPM4 ion conductivity, not within the plasma membrane, but rather in TRPM4-containing vesicles.
Full article
►▼
Show Figures
Open AccessReview
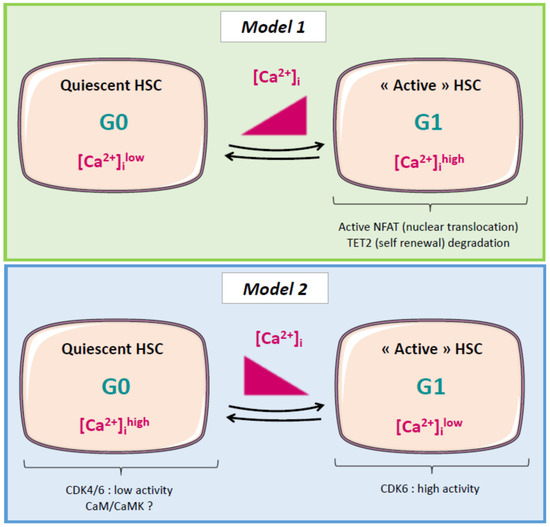
Put in a “Ca2+ll” to Acute Myeloid Leukemia
by
Clara Lewuillon, Marie-Océane Laguillaumie, Bruno Quesnel, Thierry Idziorek, Yasmine Touil and Loïc Lemonnier
Cited by 3 | Viewed by 3249
Abstract
Acute myeloid leukemia (AML) is a clonal disorder characterized by genetic aberrations in myeloid primitive cells (blasts) which lead to their defective maturation/function and their proliferation in the bone marrow (BM) and blood of affected individuals. Current intensive chemotherapy protocols result in complete
[...] Read more.
Acute myeloid leukemia (AML) is a clonal disorder characterized by genetic aberrations in myeloid primitive cells (blasts) which lead to their defective maturation/function and their proliferation in the bone marrow (BM) and blood of affected individuals. Current intensive chemotherapy protocols result in complete remission in 50% to 80% of AML patients depending on their age and the AML type involved. While alterations in calcium signaling have been extensively studied in solid tumors, little is known about the role of calcium in most hematologic malignancies, including AML. Our purpose with this review is to raise awareness about this issue and to present (i) the role of calcium signaling in AML cell proliferation and differentiation and in the quiescence of hematopoietic stem cells; (ii) the interplay between mitochondria, metabolism, and oxidative stress; (iii) the effect of the BM microenvironment on AML cell fate; and finally (iv) the mechanism by which chemotherapeutic treatments modify calcium homeostasis in AML cells.
Full article
►▼
Show Figures
Open AccessReview
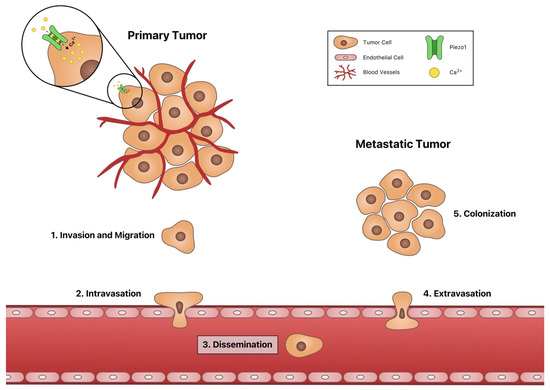
Channeling the Force: Piezo1 Mechanotransduction in Cancer Metastasis
by
Jenna A. Dombroski, Jacob M. Hope, Nicole S. Sarna and Michael R. King
Cited by 33 | Viewed by 5690
Abstract
Cancer metastasis is one of the leading causes of death worldwide, motivating research into identifying new methods of preventing cancer metastasis. Recently there has been increasing interest in understanding how cancer cells transduce mechanical forces into biochemical signals, as metastasis is a process
[...] Read more.
Cancer metastasis is one of the leading causes of death worldwide, motivating research into identifying new methods of preventing cancer metastasis. Recently there has been increasing interest in understanding how cancer cells transduce mechanical forces into biochemical signals, as metastasis is a process that consists of a wide range of physical forces. For instance, the circulatory system through which disseminating cancer cells must transit is an environment characterized by variable fluid shear stress due to blood flow. Cancer cells and other cells can transduce physical stimuli into biochemical responses using the mechanosensitive ion channel Piezo1, which is activated by membrane deformations that occur when cells are exposed to physical forces. When active, Piezo1 opens, allowing for calcium flux into the cell. Calcium, as a ubiquitous second-messenger cation, is associated with many signaling pathways involved in cancer metastasis, such as angiogenesis, cell migration, intravasation, and proliferation. In this review, we discuss the roles of Piezo1 in each stage of cancer metastasis in addition to its roles in immune cell activation and cancer cell death.
Full article
►▼
Show Figures
Open AccessArticle
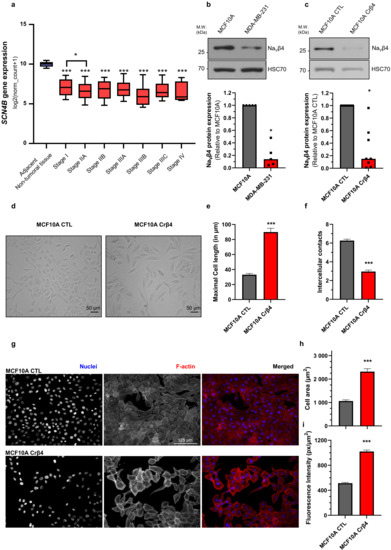
The Voltage-Gated Sodium Channel Beta4 Subunit Maintains Epithelial Phenotype in Mammary Cells
by
Adélaïde Doray, Roxane Lemoine, Marc Severin, Stéphanie Chadet, Osbaldo Lopez-Charcas, Audrey Héraud, Christophe Baron, Pierre Besson, Arnaud Monteil, Stine Falsig Pedersen and Sébastien Roger
Cited by 2 | Viewed by 2862
Abstract
The
SCN4B gene, coding for the Na
Vβ4 subunit of voltage-gated sodium channels, was recently found to be expressed in normal epithelial cells and down-regulated in several cancers. However, its function in normal epithelial cells has not been characterized. In this study,
[...] Read more.
The
SCN4B gene, coding for the Na
Vβ4 subunit of voltage-gated sodium channels, was recently found to be expressed in normal epithelial cells and down-regulated in several cancers. However, its function in normal epithelial cells has not been characterized. In this study, we demonstrated that reducing Na
Vβ4 expression in MCF10A non-cancer mammary epithelial cells generated important morphological changes observed both in two-dimensional cultures and in three-dimensional cysts. Most notably, the loss of Na
Vβ4 induced a complete loss of epithelial organisation in cysts and increased proteolytic activity towards the extracellular matrix. Loss of epithelial morphology was associated with an increased degradation of β-catenin, reduced E-cadherin expression and induction of mesenchymal markers N-cadherin, vimentin, and α-SMA expression. Overall, our results suggest that Navβ4 may participate in the maintenance of the epithelial phenotype in mammary cells and that its downregulation might be a determining step in early carcinogenesis.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
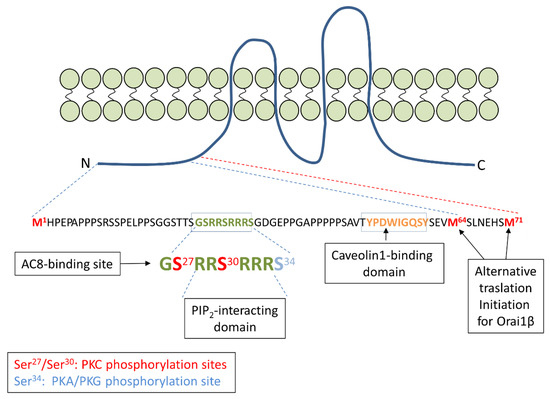
The Orai1-AC8 Interplay: How Breast Cancer Cells Escape from Orai1 Channel Inactivation
by
José Sánchez-Collado, José J. López and Juan A. Rosado
Cited by 3 | Viewed by 2682
Abstract
The interplay between the Ca
2+-sensitive adenylyl cyclase 8 (AC8) and Orai1 channels plays an important role both in the activation of the cAMP/PKA signaling and the modulation of Orai1-dependent Ca
2+ signals. AC8 interacts with a N-terminal region that is exclusive
[...] Read more.
The interplay between the Ca
2+-sensitive adenylyl cyclase 8 (AC8) and Orai1 channels plays an important role both in the activation of the cAMP/PKA signaling and the modulation of Orai1-dependent Ca
2+ signals. AC8 interacts with a N-terminal region that is exclusive to the Orai1 long variant, Orai1α. The interaction between both proteins allows the Ca
2+ that enters the cell through Orai1α to activate the generation of cAMP by AC8. Subsequent PKA activation results in Orai1α inactivation by phosphorylation at serine-34, thus shaping Orai1-mediated cellular functions. In breast cancer cells, AC8 plays a relevant role supporting a variety of cancer hallmarks, including proliferation and migration. Breast cancer cells overexpress AC8, which shifts the AC8-Orai1 stoichiometry in favor of the former and leads to the impairment of PKA-dependent Orai1α inactivation. This mechanism contributes to the enhanced SOCE observed in triple-negative breast cancer cells. This review summarizes the functional interaction between AC8 and Orai1α in normal and breast cancer cells and its relevance for different cancer features.
Full article
►▼
Show Figures