Immuno-Metabolic Crosstalk in Oncogenesis
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Immunology".
Viewed by 27775
Share This Topical Collection
Editor
 Prof. Aksam J. Merched
Prof. Aksam J. Merched
 Prof. Aksam J. Merched
Prof. Aksam J. Merched
E-Mail
Website
Collection Editor
Department of Health Sciences and Bordeaux Institute of Oncology (INSERM U1312), University of Bordeaux, 146 rue Léo Saignat, CEDEX, 33076 Bordeaux, France
Interests: metabolism; inflammation; immunosuppression; cancers; lipids; liver
Topical Collection Information
Dear colleagues,
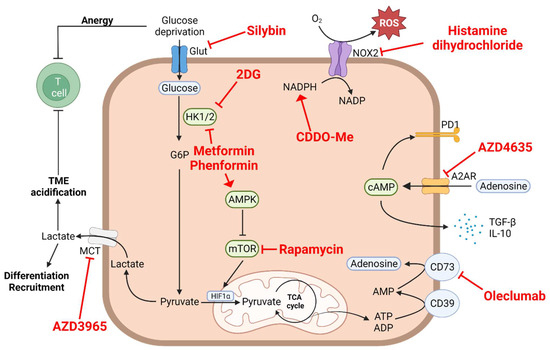
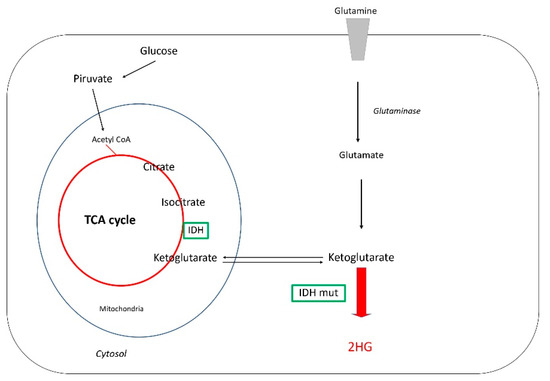
Altered cellular metabolism and immunosuppression in the tumor microenvironment became emerging hallmarks of cancer. Oncometabolites are needed to sustain tumor cell survival, growth, and proliferation by fulfilling the energetic needs and providing the raw materials and signaling molecules for many oncogenic pathways. Some are capable of driving immune cell polarization toward immunosuppressive and cancer-tolerant isotypes. The main focus of this Topical Collection is the crosstalk between these two hallmarks in the context of oncogenesis. Decoding this subtle crosstalk at the molecular and cellular levels is a prerequisite for the discovery of more effective combinatory therapeutic approaches targeting immuno-metabolism of cancers.
I am seeking all topics relevant to metabolic defect, adaptation, nutritional addiction of cancer cells, and modulation of tumor-targeted immune responses.
I am looking forward to receiving your contribution.
Prof. Aksam J. Merched
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- cancer
- immune resolution
- immunosuppression
- immunoediting
- immunotherapy
- mitochondria
- metabolism
- oncometabolites
- nutritional dependence
- epigenetics
Published Papers (9 papers)
Open AccessArticle
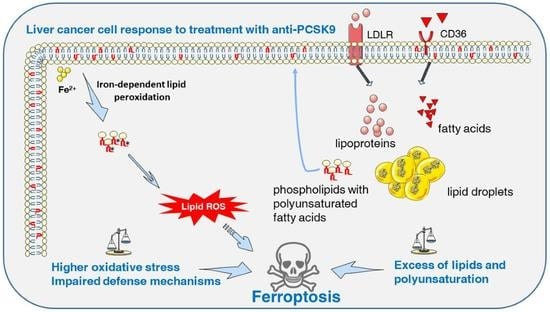
Targeting PCSK9 in Liver Cancer Cells Triggers Metabolic Exhaustion and Cell Death by Ferroptosis
by
Malak Alannan, Hala Fatrouni, Véronique Trézéguet, Franziska Dittrich-Domergue, Patrick Moreau, Géraldine Siegfried, Benjamin Liet, Abdel-Majid Khatib, Christophe F. Grosset, Bassam Badran, Hussein Fayyad-Kazan and Aksam J. Merched
Cited by 8 | Viewed by 2773
Abstract
Deregulated lipid metabolism is a common feature of liver cancers needed to sustain tumor cell growth and survival. We aim at taking advantage of this vulnerability and rewiring the oncogenic metabolic hub by targeting the key metabolic player pro-protein convertase subtilisin/kexin type 9
[...] Read more.
Deregulated lipid metabolism is a common feature of liver cancers needed to sustain tumor cell growth and survival. We aim at taking advantage of this vulnerability and rewiring the oncogenic metabolic hub by targeting the key metabolic player pro-protein convertase subtilisin/kexin type 9 (PCSK9). We assessed the effect of PCSK9 inhibition using the three hepatoma cell lines Huh6, Huh7 and HepG2 and validated the results using the zebrafish in vivo model. PCSK9 deficiency led to strong inhibition of cell proliferation in all cell lines. At the lipid metabolic level, PCSK9 inhibition was translated by an increase in intracellular neutral lipids, phospholipids and polyunsaturated fatty acids as well as a higher accumulation of lipid hydroperoxide. Molecular signaling analysis involved the disruption of the sequestome 1/Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (p62/Keap1/Nrf2) antioxidative axis, leading to ferroptosis, for which morphological features were confirmed by electron and confocal microscopies. The anti-tumoral effects of PCSK9 deficiency were validated using xenograft experiments in zebrafish. The inhibition of PCSK9 was effective in disrupting the oncometabolic process, inducing metabolic exhaustion and enhancing the vulnerability of cancer cells to iron-triggered lipid peroxidation. We provide strong evidence supporting the drug repositioning of anti-PCSK9 approaches to treat liver cancers.
Full article
►▼
Show Figures
Open AccessReview
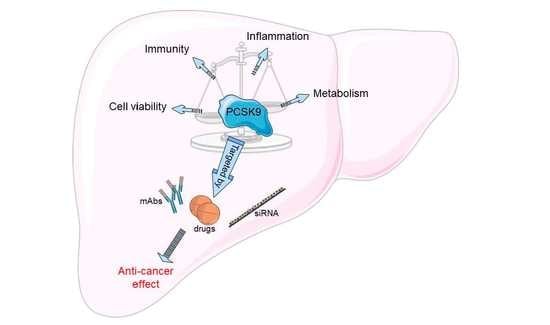
PCSK9 in Liver Cancers at the Crossroads between Lipid Metabolism and Immunity
by
Malak Alannan, Nabil G. Seidah and Aksam J. Merched
Cited by 5 | Viewed by 2867
Abstract
Metabolic rewiring and defective immune responses are considered to be the main driving forces sustaining cell growth and oncogenesis in many cancers. The atypical enzyme, proprotein convertase subtilisin/kexin type 9 (PCSK9), is produced by the liver in large amounts and plays a major
[...] Read more.
Metabolic rewiring and defective immune responses are considered to be the main driving forces sustaining cell growth and oncogenesis in many cancers. The atypical enzyme, proprotein convertase subtilisin/kexin type 9 (PCSK9), is produced by the liver in large amounts and plays a major role in lipid metabolism via the control of the low density lipoprotein receptor (LDLR) and other cell surface receptors. In this context, many clinical studies have clearly demonstrated the high efficacy of PCSK9 inhibitors in treating hyperlipidemia and cardiovascular diseases. Recent data implicated PCSK9 in the degradation of major histocompatibility complex I (MHC-I) receptors and the immune system as well as in other physiological activities. This review highlights the complex crosstalk between PCSK9, lipid metabolism and immunosuppression and underlines the latest advances in understanding the involvement of this convertase in other critical functions. We present a comprehensive assessment of the different strategies targeting PCSK9 and show how these approaches could be extended to future therapeutic options to treat cancers with a main focus on the liver.
Full article
►▼
Show Figures
Open AccessArticle
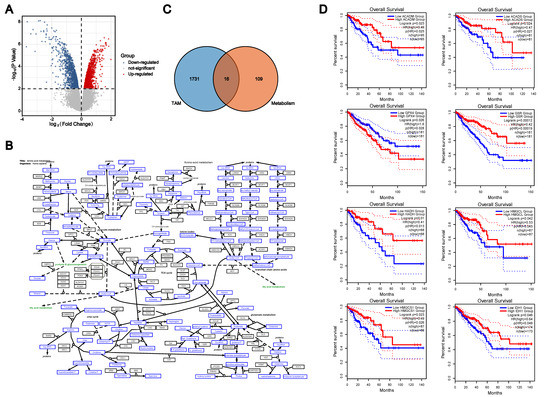
The Role of Amino Acid Metabolism of Tumor Associated Macrophages in the Development of Colorectal Cancer
by
Manman Jiang, Hongquan Cui, Zhihong Liu, Xin Zhou, Ling Zhang, Longnv Cao and Miao Wang
Cited by 8 | Viewed by 2160
Abstract
Tumor-associated macrophages (TAMs) are important immune cells in the tumor microenvironment (TME). Previous studies have shown that TAMs play a dual role in the development of colorectal cancer and promote the additional exploration of the immune escape of colorectal cancer. Studies have confirmed
[...] Read more.
Tumor-associated macrophages (TAMs) are important immune cells in the tumor microenvironment (TME). Previous studies have shown that TAMs play a dual role in the development of colorectal cancer and promote the additional exploration of the immune escape of colorectal cancer. Studies have confirmed that macrophages utilize amino acid metabolism under the stimulation of some factors released by tumor cells, thus affecting the direction of polarization. Therefore, we investigated the effect of amino acid metabolism on macrophage function and the involved mechanism. Based on the comprehensive analysis of the GSE18804 GEO dataset and amino acid metabolism pathway, we identified the eight key enzymes of amino acid metabolism in colon TAMs, namely, ACADM, ACADS, GPX4, GSR, HADH, HMGCL, HMGCS1 and IDH1. We then evaluated the expression, survival analysis and relationship of clinicopathological features with these eight key enzymes. The results supported the critical role of these eight genes in colorectal cancer. Macrophages phagocytose tumor cells, and these eight key enzymes were identified in combination with GPX4, a critical protein of ferroptosis, suggesting that the change in the expression of these eight key enzymes in TAMs may be involved in the regulation of colorectal cancer through cell death. Correlation analysis of three programmed cell death (PCD) marker genes indicated that these eight key enzymes may cause macrophage death through pyroptosis, leading to immune escape of colorectal cancer. We also investigated the regulation of ACADS in CRC using flow cytometry, qPCR and ELISAs, which demonstrated that an ACADS deficiency polarizes TAMs to M2 macrophages. In summary, the present study revealed the relationship between amino acid metabolism and the cell death of macrophages, providing a new research direction for the molecular mechanism of macrophage polarization.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
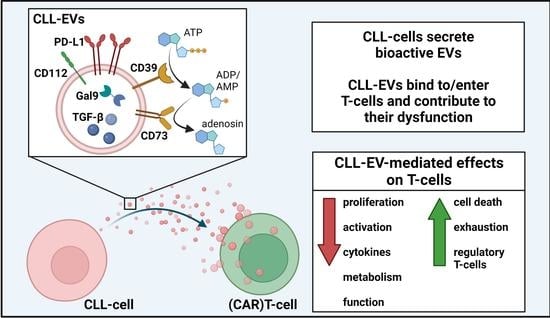
CLL-Derived Extracellular Vesicles Impair T-Cell Activation and Foster T-Cell Exhaustion via Multiple Immunological Checkpoints
by
Martin Böttcher, Romy Böttcher-Loschinski, Sascha Kahlfuss, Michael Aigner, Andreas Gießl, Andreas Mackensen, Ursula Schlötzer-Schrehardt, Thomas Tüting, Heiko Bruns and Dimitrios Mougiakakos
Cited by 8 | Viewed by 3324
Abstract
Background: Chronic lymphocytic leukemia (CLL) is characterized by the clonal expansion of malignant B-cells and multiple immune defects. This leads, among others, to severe infectious complications and inefficient immune surveillance. T-cell deficiencies in CLL include enhanced immune(-metabolic) exhaustion, impaired activation and cytokine production,
[...] Read more.
Background: Chronic lymphocytic leukemia (CLL) is characterized by the clonal expansion of malignant B-cells and multiple immune defects. This leads, among others, to severe infectious complications and inefficient immune surveillance. T-cell deficiencies in CLL include enhanced immune(-metabolic) exhaustion, impaired activation and cytokine production, and immunological synapse malformation. Several studies have meanwhile reported CLL-cell–T-cell interactions that culminate in T-cell dysfunction. However, the complex entirety of their interplay is incompletely understood. Here, we focused on the impact of CLL cell-derived vesicles (EVs), which are known to exert immunoregulatory effects, on T-cell function.
Methods: We characterized EVs secreted by CLL-cells and determined their influence on T-cells in terms of survival, activation, (metabolic) fitness, and function.
Results: We found that CLL-EVs hamper T-cell viability, proliferation, activation, and metabolism while fostering their exhaustion and formation of regulatory T-cell subsets. A detailed analysis of the CLL-EV cargo revealed an abundance of immunological checkpoints (ICs) that could explain the detected T-cell dysregulations.
Conclusions: The identification of a variety of ICs loaded on CLL-EVs may account for T-cell defects in CLL patients and could represent a barrier for immunotherapies such as IC blockade or adoptive T-cell transfer. Our findings could pave way for improving antitumor immunity by simultaneously targeting EV formation or multiple ICs.
Full article
►▼
Show Figures
Open AccessReview
Influence of the Metabolism on Myeloid Cell Functions in Cancers: Clinical Perspectives
by
Thomas Boyer, Céline Blaye, Nicolas Larmonier and Charlotte Domblides
Cited by 1 | Viewed by 2568
Abstract
Tumor metabolism plays a crucial role in sustaining tumorigenesis. There have been increasing reports regarding the role of tumor metabolism in the control of immune cell functions, generating a potent immunosuppressive contexture that can lead to immune escape. The metabolic reprogramming of tumor
[...] Read more.
Tumor metabolism plays a crucial role in sustaining tumorigenesis. There have been increasing reports regarding the role of tumor metabolism in the control of immune cell functions, generating a potent immunosuppressive contexture that can lead to immune escape. The metabolic reprogramming of tumor cells and the immune escape are two major hallmarks of cancer, with several instances of crosstalk between them. In this paper, we review the effects of tumor metabolism on immune cells, focusing on myeloid cells due to their important role in tumorigenesis and immunosuppression from the early stages of the disease. We also discuss ways to target this specific crosstalk in cancer patients.
Full article
►▼
Show Figures
Open AccessReview
Glioblastoma: Relationship between Metabolism and Immunosuppressive Microenvironment
by
Ainhoa Hernández, Marta Domènech, Ana M. Muñoz-Mármol, Cristina Carrato and Carmen Balana
Cited by 14 | Viewed by 3927
Abstract
Glioblastoma (GBM) is the most aggressive brain tumor in adults and is characterized by an immunosuppressive microenvironment. Different factors shaping this tumor microenvironment (TME) regulate tumor initiation, progression, and treatment response. Genetic alterations and metabolism pathways are two main elements that influence tumor
[...] Read more.
Glioblastoma (GBM) is the most aggressive brain tumor in adults and is characterized by an immunosuppressive microenvironment. Different factors shaping this tumor microenvironment (TME) regulate tumor initiation, progression, and treatment response. Genetic alterations and metabolism pathways are two main elements that influence tumor immune cells and TME. In this manuscript, we review how both factors can contribute to an immunosuppressive state and overview the strategies being tested.
Full article
►▼
Show Figures
Open AccessReview
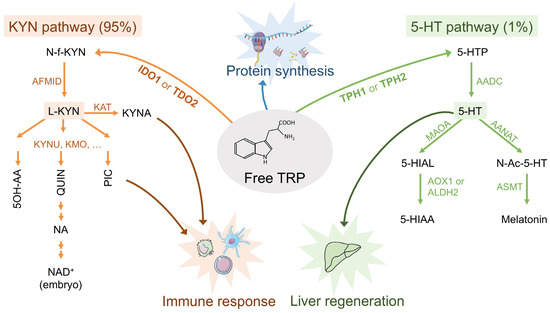
Immuno-Metabolic Modulation of Liver Oncogenesis by the Tryptophan Metabolism
by
Véronique Trézéguet, Hala Fatrouni and Aksam J. Merched
Cited by 6 | Viewed by 2995
Abstract
Metabolic rewiring in tumor cells is a major hallmark of oncogenesis. Some of the oncometabolites drive suppressive and tolerogenic signals from the immune system, which becomes complicit to the advent and the survival of neoplasia. Tryptophan (TRP) catabolism through the kynurenine (KYN) pathway
[...] Read more.
Metabolic rewiring in tumor cells is a major hallmark of oncogenesis. Some of the oncometabolites drive suppressive and tolerogenic signals from the immune system, which becomes complicit to the advent and the survival of neoplasia. Tryptophan (TRP) catabolism through the kynurenine (KYN) pathway was reported to play immunosuppressive actions across many types of cancer. Extensive debate of whether the culprit of immunosuppression was the depletion of TRP or rather KYN accumulation in the tumor microenvironment has been ongoing for years. Results from clinical trials assessing the benefit of inhibiting key limiting enzymes of this pathway such as indoleamine 2,3-dioxygenase (IDO1) or tryptophan 2,3-dioxygenase (TDO2) failed to meet the expectations. Bearing in mind the complexity of the tumoral terrain and the existence of different cancers with
IDO1/TDO2 expressing and non-expressing tumoral cells, here we present a comprehensive analysis of the TRP global metabolic hub and the driving potential of the process of oncogenesis with the main focus on liver cancers.
Full article
►▼
Show Figures
Open AccessReview
The Metabolic Control of Myeloid Cells in the Tumor Microenvironment
by
Eloise Ramel, Sebastian Lillo, Boutaina Daher, Marina Fioleau, Thomas Daubon and Maya Saleh
Cited by 4 | Viewed by 3525
Abstract
Myeloid cells are a key determinant of tumor progression and patient outcomes in a range of cancers and are therefore being actively pursued as targets of new immunotherapies. The recent use of high-dimensional single-cell approaches, e.g., mass cytometry and single-cell RNA-sequencing (scRNA-seq) has
[...] Read more.
Myeloid cells are a key determinant of tumor progression and patient outcomes in a range of cancers and are therefore being actively pursued as targets of new immunotherapies. The recent use of high-dimensional single-cell approaches, e.g., mass cytometry and single-cell RNA-sequencing (scRNA-seq) has reinforced the predominance of myeloid cells in the tumor microenvironment and uncovered their phenotypic diversity in different cancers. The cancerous metabolic environment has emerged as a critical modulator of myeloid cell functions in anti-tumor immunity versus immune suppression and immune evasion. Here, we discuss mechanisms of immune-metabolic crosstalk in tumorigenesis, with a particular focus on the tumor-associated myeloid cell’s metabolic programs. We highlight the impact of several metabolic pathways on the pro-tumoral functions of tumor-associated macrophages and myeloid-derived suppressor cells and discuss the potential myeloid cell metabolic checkpoints for cancer immunotherapy, either as monotherapies or in combination with other immunotherapies.
Full article
►▼
Show Figures
Open AccessCommunication
Alteration of Tissue Marking Dyes Depends on Used Chromogen during Immunohistochemistry
by
Selina Kiefer, Julia Huber, Hannah Füllgraf, Kristin Sörensen, Agnes Csanadi, Maren Nicole Stillger, Martin Werner, Hans-Eckart Schaefer, Peter Bronsert and Konrad Aumann
Cited by 1 | Viewed by 2160
Abstract
Pathological biopsy protocols require tissue marking dye (TMD) for orientation. In some cases (e.g., close margin), additional immunohistochemical analyses can be necessary. Therefore, the correlation between the applied TMD during macroscopy and the examined TMD during microscopy is crucial for the correct orientation,
[...] Read more.
Pathological biopsy protocols require tissue marking dye (TMD) for orientation. In some cases (e.g., close margin), additional immunohistochemical analyses can be necessary. Therefore, the correlation between the applied TMD during macroscopy and the examined TMD during microscopy is crucial for the correct orientation, the residual tumour status and the subsequent therapeutic regime. In this context, our group observed colour changes during routine immunohistochemistry. Tissue specimens were marked with various TMD and processed by two different methods. TMD (blue, red, black, yellow and green) obtained from three different providers (A, B and C, and Whiteout/Tipp-Ex
®) were used. Immunohistochemistry was performed manually via stepwise omission of reagents to identify the colour changing mechanism. Blue colour from provider A changed during immunohistochemistry into black, when 3,3′-Diaminobenzidine-tetrahydrochloride-dihydrate (DAB) and H
2O
2 was applied as an immunoperoxidase-based terminal colour signal. No other applied reagents, nor tissue texture or processing showed any influence on the colour. The remaining colours from provider A and the other colours did not show any changes during immunohistochemistry. Our results demonstrate an interesting and important pitfall in routine immunohistochemistry-based diagnostics that pathologists should be aware of. Furthermore, the chemical rationale behind the observed misleading colour change is discussed.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: PCSK9 in liver cancers at the crossroad between lipid metabolism and immunity
Authors: Malak Alannan1, Nabil G. Seidah2 and Aksam J. Merched1,
Affiliation: 1 Bordeaux University, 33000, Bordeaux, France: Inserm U 1312, Bordeaux Institute of Oncology, BRIC; 146 rue Léo Saignat, 33076 Bordeaux, France and 2 Laboratory of Biochemical Neuroendocrinology, Montreal Clinical Research Institute (IRCM, affiliated to the University of Montreal), Montreal, QC, H2W 1R7, Canada
Abstract: Metabolic rewiring and defective immune responses are considered as the main driving forces to sustain cell growth and oncogenesis in many cancers. The atypical enzyme, proprotein convertase subtilisin/kexin type 9 or PCSK9, is highly produced by the liver and plays a major role in lipid metabolism via the control of the LDL receptor and other cell surface receptors. In this context, many clinical studies clearly demonstrated the high efficacy of PCSK9 inhibitors in treating hyperlipidemia and cardiovascular diseases. Recent data implicated PCSK9 in the degradation of MHC-I receptors and the immune system as well as in other housekeeping activities. This review highlights the complex crosstalk between PCSK9, lipid metabolism and immunosuppression and underlines the latest advances in understanding the involvement of this enzyme in other critical functions. We present a comprehensive assessment of the different strategies targeting PCSK9 and show how these approaches could be extended to future therapeutic options to treat cancers with main focus