Hypoxia-Inducible Factors in Human Physiology and Diseases
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 16039Editors
Interests: hypoxia; HIF; Epigenetics; metabolism; noncoding RNA
Topical Collection Information
Dear Colleagues,
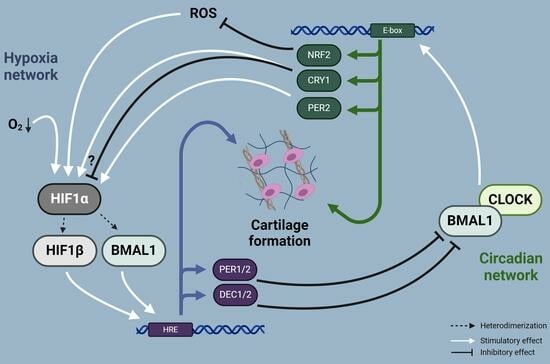
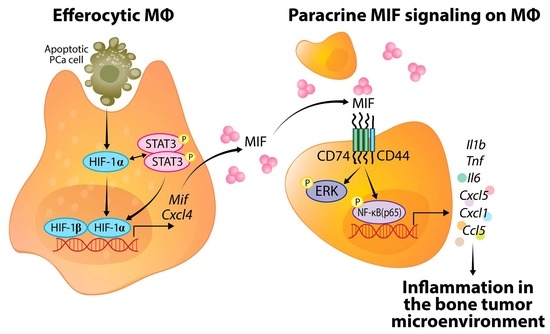
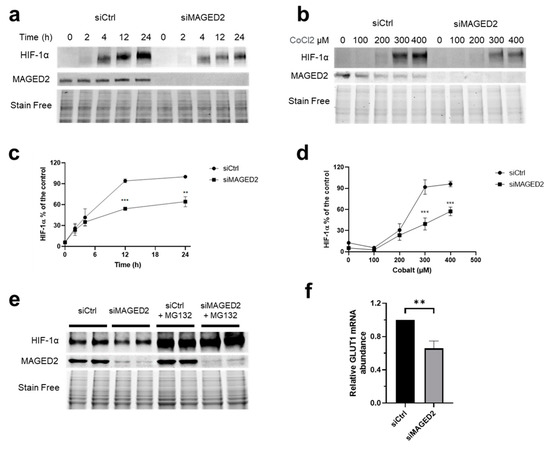
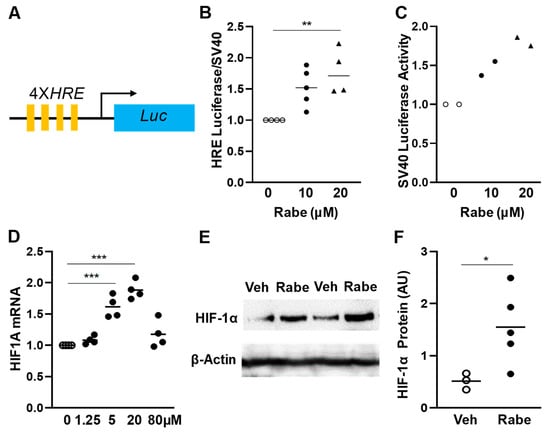
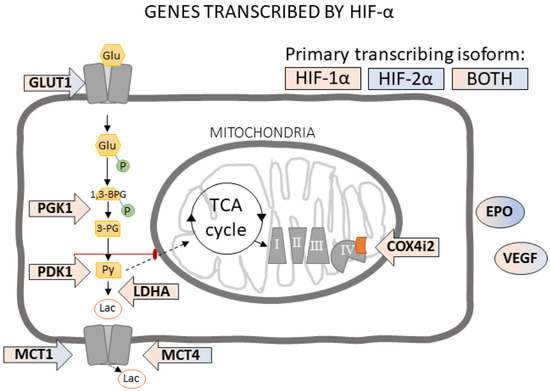
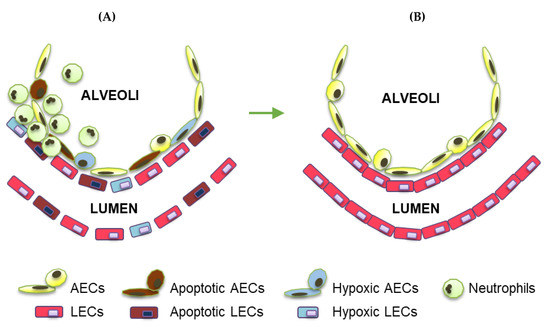
Oxygen (O2) is the third abundant element in the universe and essential for life in all metazoans. It is required for ATP production in the mitochondria, which drives many physiological processes in the living cell. Reduced availability of O2 (also known as hypoxia) is associated with many human diseases, including cancer, stroke, anemia, sleep apnea, wound healing, myocardial infarction, and infection. The hypoxia response is primarily controlled by hypoxia-inducible factor, a family of basic Helix-Loop-Helix (bHLH)-Per-Arnt-Sim (PAS) transcription factors consisting of an O2-regulated α subunit and a constitutively expressed β subunit. Over the past three decades, HIF has been shown to mediate many physiological and pathological processes, including development, angiogenesis, erythropoiesis, energy metabolism, epigenetic reprogramming, cell survival, cell motility, stemness, inflammation, metastasis, and neurodegeneration. Despite the remarkable advancement in the HIF research filed, it remains poorly understood how HIF regulates physiological processes and drives disease progression.
The purpose of the Topical Collection on “Hypoxia-Inducible Factors in Human Physiology and Diseases” is to discuss new developments of HIF-dependent physiological and pathological processes. We welcome both original research articles and reviews that address our focused topic, with the goal of advancing the fundamental knowledge of HIF biology in human physiology and diseases.
Dr. Weibo Luo
Dr. Yingfei Wang
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- hypoxia
- HIF
- transcriptional activity
- inflammation
- metabolism
- immune evasion
- metastasis
- tissue repair and regeneration
- neurodegeneration