The Heparanase/Heparanase-2 Regulatory Network in Health and Disease
A project collection of Cells (ISSN 2073-4409). This project collection belongs to the section "Cell Microenvironment".
Papers displayed on this page all arise from the same project. Editorial decisions were made independently of project staff and handled by the Editor-in-Chief or qualified Editorial Board members.
Viewed by 13468
Share This Project Collection
Editors
 Prof. Dr. Israël Vlodavsky
Prof. Dr. Israël Vlodavsky
 Prof. Dr. Israël Vlodavsky
Prof. Dr. Israël Vlodavsky
E-Mail
Website
Guest Editor
Rappaport Faculty of Medicine, Technion Integrated Cancer Center (TICC), Technion—Israel Institute of Technology, Haifa 31096, Israel
Interests: heparanase; tumor microenvironment; extracellular matrix; tumor progression; metastasis
 Prof. Dr. Jin-Ping Li
Prof. Dr. Jin-Ping Li
 Prof. Dr. Jin-Ping Li
Prof. Dr. Jin-Ping Li
E-Mail
Website
Co-Guest Editor
SciLifeLab Uppsala, The Biomedical Center, Department of Medical Biochemistry and Microbiology, University of Uppsala, 75237 Uppsala, Sweden
Interests: heparanase; tumor microenvironment
Project Overview
Dear Colleagues,
Heparanase is an endoglycosidase that cleaves the heparan sulfate (HS) side chains of HS proteoglycans (HSPG). Compelling evidence ties heparanase to all aspects of cancer progression, namely, tumor initiation, growth, metastasis, and chemoresistance, supporting the notion that heparanase is a master regulator of cancer aggressiveness and crosstalk with the tumor microenvironment. Heparanase upregulation has also been identified in inflammatory diseases, atherosclerosis, fibrosis, diabetes, diabetic nephropathy, and viral infection.
Unlike the intense research effort devoted to exploring the significance of heparanase in human diseases, very little attention has been given to heparanase 2 (=Hpa2), a close homolog of heparanase that lacks HS-degrading activity. Notably, Hpa2 retains the capacity to bind heparin/HS and, importantly, exhibits an even higher affinity toward HS than heparanase, thus competing for HS binding and inhibiting heparanase enzymatic activity. Hpa2 exhibits antitumor, anti-angiogenic and anti-inflammatory protective effects due in part to its heparanase-inhibiting activity, and to an elevation of endoplasmic reticulum (ER) stress that leads to apoptotic cell death. It appears that Hpa2 functions as a natural inhibitor of the heparanase enzyme and that the heparanase/Hpa2 ratio regulates to a large extent the balance between disease progression and suppression.
This Special Issue aims at exploring the multifaceted activities of heparanase and heparanase-2 in cancer and inflammation and the related clinical implications and future directions.
Prof. Dr. Israël Vlodavsky
Prof. Dr. Jin-Ping Li
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- heparanase
- tumor microenvironment
- extracellular matrix
- tumor progression
- metastasis
Published Papers (7 papers)
Open AccessArticle
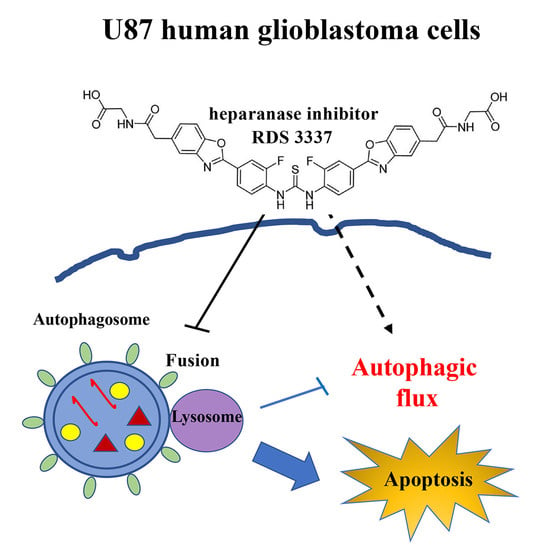
Role of a Novel Heparanase Inhibitor on the Balance between Apoptosis and Autophagy in U87 Human Glioblastoma Cells
by
Valeria Manganelli, Roberta Misasi, Gloria Riitano, Antonella Capozzi, Vincenzo Mattei, Tuba Rana Caglar, Davide Ialongo, Valentina Noemi Madia, Antonella Messore, Roberta Costi, Roberto Di Santo, Maurizio Sorice and Tina Garofalo
Viewed by 1143
Abstract
Background: Heparanase (HPSE) is an endo-β-glucuronidase that cleaves heparan sulfate side chains, leading to the disassembly of the extracellular matrix, facilitating cell invasion and metastasis dissemination. In this research, we investigated the role of a new HPSE inhibitor, RDS 3337, in the regulation
[...] Read more.
Background: Heparanase (HPSE) is an endo-β-glucuronidase that cleaves heparan sulfate side chains, leading to the disassembly of the extracellular matrix, facilitating cell invasion and metastasis dissemination. In this research, we investigated the role of a new HPSE inhibitor, RDS 3337, in the regulation of the autophagic process and the balance between apoptosis and autophagy in U87 glioblastoma cells. Methods: After treatment with RDS 3337, cell lysates were analyzed for autophagy and apoptosis-related proteins by Western blot. Results: We observed, firstly, that LC3II expression increased in U87 cells incubated with RDS 3337, together with a significant increase of p62/SQSTM1 levels, indicating that RDS 3337 could act through the inhibition of autophagic-lysosomal flux of LC3-II, thereby leading to accumulation of lipidated LC3-II form. Conversely, the suppression of autophagic flux could activate apoptosis mechanisms, as revealed by the activation of caspase 3, the increased level of cleaved Parp1, and DNA fragmentation. Conclusions: These findings support the notion that HPSE promotes autophagy, providing evidence that RDS 3337 blocks autophagic flux. It indicates a role for HPSE inhibitors in the balance between apoptosis and autophagy in U87 human glioblastoma cells, suggesting a potential role for this new class of compounds in the control of tumor growth progression.
Full article
►▼
Show Figures
Open AccessArticle
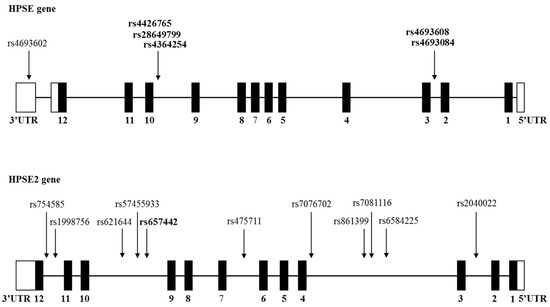
Effect of HPSE and HPSE2 SNPs on the Risk of Developing Primary Paraskeletal Multiple Myeloma
by
Olga Ostrovsky, Katia Beider, Hila Magen, Merav Leiba, Ralph D. Sanderson, Israel Vlodavsky and Arnon Nagler
Viewed by 1342
Abstract
Multiple myeloma (MM) is a plasma cell malignancy that is accompanied by hypercalcemia, renal failure, anemia, and lytic bone lesions. Heparanase (HPSE) plays an important role in supporting and promoting myeloma progression, maintenance of plasma cell stemness, and resistance to therapy. Previous studies
[...] Read more.
Multiple myeloma (MM) is a plasma cell malignancy that is accompanied by hypercalcemia, renal failure, anemia, and lytic bone lesions. Heparanase (HPSE) plays an important role in supporting and promoting myeloma progression, maintenance of plasma cell stemness, and resistance to therapy. Previous studies identified functional single nucleotide polymorphisms (SNPs) located in the HPSE gene. In the present study, 5 functional HPSE SNPs and 11 novel HPSE2 SNPs were examined. A very significant association between two enhancer (rs4693608 and rs4693084), and two insulator (rs4364254 and rs4426765) HPSE SNPs and primary paraskeletal disease (PS) was observed. SNP rs657442, located in intron 9 of the HPSE2 gene, revealed a significant protective association with primary paraskeletal disease and lytic bone lesions. The present study demonstrates a promoting (HPSE gene) and protective (HPSE2 gene) role of gene regulatory elements in the development of paraskeletal disease and bone morbidity. The effect of signal discrepancy between myeloma cells and normal cells of the tumor microenvironment is proposed as a mechanism for the involvement of heparanase in primary PS. We suggest that an increase in heparanase-2 expression can lead to effective suppression of heparanase activity in multiple myeloma accompanied by extramedullary and osteolytic bone disease.
Full article
►▼
Show Figures
Open AccessArticle
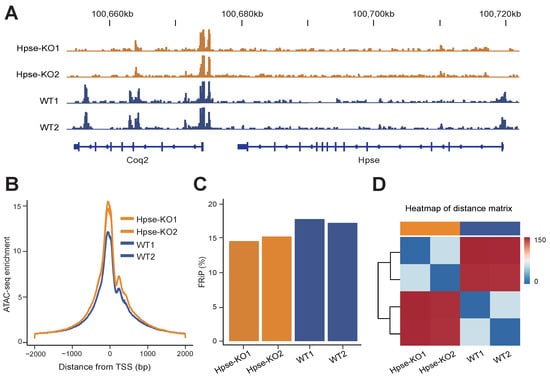
Heparanase Modulates Chromatin Accessibility
by
Honglian Li, Hua Zhang, Amelie Wenz, Ziqi Kang, Helen Wang, Israel Vlodavsky, Xingqi Chen and Jinping Li
Cited by 1 | Viewed by 1677
Abstract
Heparanase is the sole endoglucuronidase that degrades heparan sulfate in the cell surface and extracellular matrix (ECM). Several studies have reported the localization of heparanase in the cell nucleus, but the functional role of the nuclear enzyme is still obscure. Subjecting mouse embryonic
[...] Read more.
Heparanase is the sole endoglucuronidase that degrades heparan sulfate in the cell surface and extracellular matrix (ECM). Several studies have reported the localization of heparanase in the cell nucleus, but the functional role of the nuclear enzyme is still obscure. Subjecting mouse embryonic fibroblasts (MEFs) derived from heparanase knockout (Hpse-KO) mice and applying transposase-accessible chromatin with sequencing (ATAC-seq), we revealed that heparanase is involved in the regulation of chromatin accessibility. Integrating with genome-wide analysis of chromatin states revealed an overall low activity in the enhancer and promoter regions of Hpse-KO MEFs compared with wild-type (WT) MEFs. Western blot analysis of MEFs and tissues derived from Hpse-KO vs. WT mice confirmed reduced expression of H3K27ac (acetylated lysine at N-terminal position 27 of the histone H3 protein). Our results offer a mechanistic explanation for the well-documented attenuation of inflammatory responses and tumor growth in Hpse-KO mice.
Full article
►▼
Show Figures
Open AccessReview
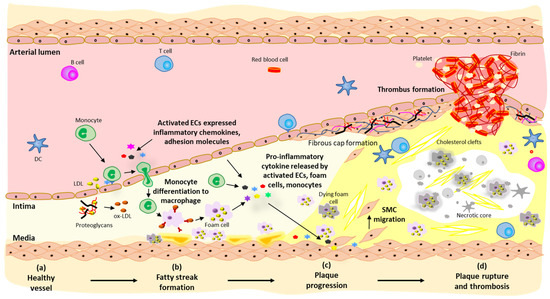
Heparanase: A Novel Therapeutic Target for the Treatment of Atherosclerosis
by
Tien K. Nguyen, Stephanie Paone, Enoch Chan, Ivan K. H. Poon, Amy A. Baxter, Shane R. Thomas and Mark D. Hulett
Cited by 9 | Viewed by 2515
Abstract
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide, and its management places a huge burden on healthcare systems through hospitalisation and treatment. Atherosclerosis is a chronic inflammatory disease of the arterial wall resulting in the formation of lipid-rich, fibrotic
[...] Read more.
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide, and its management places a huge burden on healthcare systems through hospitalisation and treatment. Atherosclerosis is a chronic inflammatory disease of the arterial wall resulting in the formation of lipid-rich, fibrotic plaques under the subendothelium and is a key contributor to the development of CVD. As such, a detailed understanding of the mechanisms involved in the development of atherosclerosis is urgently required for more effective disease treatment and prevention strategies. Heparanase is the only mammalian enzyme known to cleave heparan sulfate of heparan sulfate proteoglycans, which is a key component of the extracellular matrix and basement membrane. By cleaving heparan sulfate, heparanase contributes to the regulation of numerous physiological and pathological processes such as wound healing, inflammation, tumour angiogenesis, and cell migration. Recent evidence suggests a multifactorial role for heparanase in atherosclerosis by promoting underlying inflammatory processes giving rise to plaque formation, as well as regulating lesion stability. This review provides an up-to-date overview of the role of heparanase in physiological and pathological processes with a focus on the emerging role of the enzyme in atherosclerosis.
Full article
►▼
Show Figures
Open AccessArticle
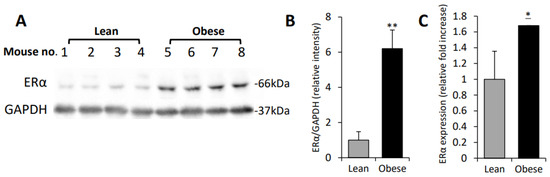
Macrophages Upregulate Estrogen Receptor Expression in the Model of Obesity-Associated Breast Carcinoma
by
Daniela Nahmias Blank, Esther Hermano, Amir Sonnenblick, Ofra Maimon, Ariel M. Rubinstein, Emmy Drai, Bella Maly, Israel Vlodavsky, Aron Popovtzer, Tamar Peretz, Amichay Meirovitz and Michael Elkin
Cited by 5 | Viewed by 1948
Abstract
Breast cancer (BC) and obesity are two heterogeneous conditions with a tremendous impact on health. BC is the most commonly diagnosed neoplasm and the leading cause of cancer-related mortality among women, and the prevalence of obesity in women worldwide reaches pandemic proportions. Obesity
[...] Read more.
Breast cancer (BC) and obesity are two heterogeneous conditions with a tremendous impact on health. BC is the most commonly diagnosed neoplasm and the leading cause of cancer-related mortality among women, and the prevalence of obesity in women worldwide reaches pandemic proportions. Obesity is a significant risk factor for both incidence and worse prognosis in estrogen receptor positive (ER+) BC. Yet, the mechanisms underlying the association between excess adiposity and increased risk/therapy resistance/poorer outcome of ER+, but not ER−negative (ER−), BC are not fully understood. Tumor-promoting action of obesity, predominantly in ER + BC patients, is often attributed to the augmented production of estrogen in ‘obese’ adipose tissue. However, in addition to the estrogen production, expression levels of ER represent a key determinant in hormone-driven breast tumorigenesis and therapy response. Here, utilizing in vitro and in vivo models of BC, we show that macrophages, whose adverse activation by obesogenic substances is fueled by heparanase (extracellular matrix-degrading enzyme), are capable of upregulating ER expression in tumor cells, in the setting of obesity-associated BC. These findings underscore a previously unknown mechanism through which interplay between cellular/extracellular elements of obesity-associated BC microenvironment influences estrogen sensitivity—a critical component in hormone-related cancer progression and resistance to therapy.
Full article
►▼
Show Figures
Open AccessArticle
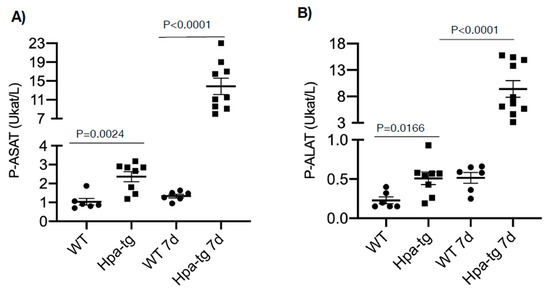
Heparanase Expression Propagates Liver Damage in CCL4-Induced Mouse Model
by
Xiaowen Cheng, Juan Jia, Tianji Zhang, Xiao Zhang, Israel Vlodavsky and Jin-ping Li
Cited by 1 | Viewed by 1815
Abstract
Heparanase is elevated in various pathological conditions, primarily cancer and inflammation. To investigate the significance and involvement of heparanase in liver fibrosis, we compared the susceptibility of wild-type (WT) and heparanase-overexpressing transgenic (Hpa-tg) mice to carbon tetrachloride (CCL4)-induced fibrosis. In comparison with WT
[...] Read more.
Heparanase is elevated in various pathological conditions, primarily cancer and inflammation. To investigate the significance and involvement of heparanase in liver fibrosis, we compared the susceptibility of wild-type (WT) and heparanase-overexpressing transgenic (Hpa-tg) mice to carbon tetrachloride (CCL4)-induced fibrosis. In comparison with WT mice, Hpa-tg mice displayed a severe degree of tissue damage and fibrosis, including higher necrotic tendency and intensified expression of smooth muscle actin. While damage to the WT liver started to recover after the acute phase, damage to the Hpa-tg liver was persistent. Recovery was attributed, in part, to heparanase-stimulated autophagic activity in response to CCL4, leading to increased apoptosis and necrosis. The total number of stellate cells was significantly higher in the Hpa-tg than the WT liver, likely contributing to the increased amounts of lipid droplets and smooth muscle actin. Our results support the notion that heparanase enhances inflammatory responses, and hence may serve as a target for the treatment of liver damage and fibrosis.
Full article
►▼
Show Figures
Open AccessArticle
CMV Seropositive Status Increases Heparanase SNPs Regulatory Activity, Risk of Acute GVHD and Yield of CD34+ Cell Mobilization
by
Olga Ostrovsky, Katia Beider, Yan Morgulis, Nira Bloom, Angel Cid-Arregui, Avichai Shimoni, Israel Vlodavsky and Arnon Nagler
Cited by 2 | Viewed by 2224
Abstract
Heparanase is an endo-β-glucuronidase that is best known for its pro-cancerous effects but is also implicated in the pathogenesis of various viruses. Activation of heparanase is a common strategy to increase viral spread and trigger the subsequent inflammatory cascade. Using a Single Nucleotide
[...] Read more.
Heparanase is an endo-β-glucuronidase that is best known for its pro-cancerous effects but is also implicated in the pathogenesis of various viruses. Activation of heparanase is a common strategy to increase viral spread and trigger the subsequent inflammatory cascade. Using a Single Nucleotide Polymorphisms (SNP)-associated approach we identified enhancer and insulator regions that regulate HPSE expression. Although a role for heparanase in viral infection has been noticed, the impact of HPSE functional SNPs has not been determined. We investigated the effect of cytomegalovirus (CMV) serostatus on the involvement of HPSE enhancer and insulator functional SNPs in the risk of acute graft versus host disease (GVHD) and granulocyte-colony stimulating factor related CD34
+ mobilization. A significant correlation between the C alleles of insulator rs4364254 and rs4426765 and CMV seropositivity was found in healthy donors and patients with hematological malignancies. The risk of developing acute GVHD after hematopoietic stem cell transplantation was identified only in CMV-seropositive patients. A significant correlation between the enhancer rs4693608 and insulator rs28649799 and CD34
+ cell mobilization was demonstrated in the CMV-seropositive donors. It is thus conceivable that latent CMV infection modulates heparanase regulatory regions and enhances the effect of functional SNPs on heparanase function in normal and pathological processes.
Full article
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: In vivo transduction of HPSE2 and LRIG2, genes implicated in urofacial syndrome, into the mouse bladder
Authors: Filipa M Lopes1, Celine Grenier1, Benjamin W Jarvis1, Adrian Lene1, Simon N Waddington2, Adrian Woolf1, Neil Roberts1
Affiliation: 1. Division of Cell Matrix Biology and Regenerative Medicine, School of Biological Sciences, The University of Manchester
2. Maternal & Fetal Medicine, EGA Institute for Women's Health, Faculty of Population Health Sciences, UCL
Abstract: Background. HPSE2, coding for heparanase 2, is a heparan sulfate binding protein with high sequence conservation with the endoglycosidase heparanase, but lacking enzymatic function. Causative pathogenic variants have been reported in HPSE2 in some patients with urofacial syndrome (UFS), a devastating early-onset bladder disease. Pathogenic variants have also been discovered in leucine-rich repeats and immunoglobulin-like domains 2 (LRIG2), in other individuals with UFS. Mouse models of both forms of UFS indicate an underlying neuropathy in the bladder. Knowing the implicated genes and the affected tissue opens the possibility of using gene therapy to cure mouse models of the disease. In this study, we aim to take the first steps to developing a treatment for UFS, by using viral vectors to deliver healthy human copies of HPSE2 and LRIG2 to neurons in the mouse bladder.
Methods. Vectors contained either a nerve-specific synapsin (SYN1) promoter or a global CAG promoter to drive human HPSE2 or LRIG2 expression. Adeno-associated virus 9 (AAV9) vector was tested in N2a neuroblastoma cells. In vivo delivery into wildtype neonatal mice was achieved by superficial temporal vein injection. Transgene expression was detected using Basescope on sectioned bladders from injected and control mice, to detect either vector transduction or transgene expression.
Results. Therapeutic human transgenes were successfully transduced into neuroblastoma cells by the AAV vectors. Next, vectors were delivered to wildtype mice by systemic injection. Mice tolerated vector dose escalation without loss of viability, weight and behaviour remaining normal to adulthood. Bladder body and outflow were analysed for endogenous and human transgene transcripts and the viral genome. The human transgenes were detected in mice injected with the relevant vector, and transcript abundance in bladder neurons correlated with the vector dose. At low vector dose, sparse human HPSE2 transcripts were observed in bladder neuron cell bodies. At high dose, abundant transcripts were observed in the autonomic nerve cell bodies, and also greater intensity of staining was observed in the bladder smooth muscle detrusor. Similarly, at low dose the SYN1 promoter drove low levels of human LRIG2 in bladder neurons, while at high dose abundant neuronal LRIG2 was detected. For other relevant organs, in the liver, highly abundance vector was detected, correlating with transgene expression. In the kidney, low or absent vector and transgene expression were observed.
Summary. Here, we present initial findings for the first study of vector mediated gene therapy for a disease congenital that affects the lower urinary tract. We provide, to our knowledge, the first ever description of transduction and therapeutic transgene expression in autonomic nerves. We confirm that our AAV9-transgene vectors are able to safely transduce bladder neurons and drive expression of HPSE2 and LRIG2. Next, we will utilise this strategy in mutant mice to ameliorate the voiding dysfunction and causative neurophysiological defects. If successful, we aim to translate this to clinical use, and intend for our strategy will become a template for other models of genetic bladder disease.
Highlights. We show, for the first time, that adeno viral vectors are able to delivery therapeutic human transgenes to autonomic nerves in the mouse bladder. This opens the possibility of using gene therapy to treat genetic diseases of the urinary tract such as urofacial syndrome, which is caused by mutations in HPSE2 and LRIG2.